Answered step by step
Verified Expert Solution
Question
1 Approved Answer
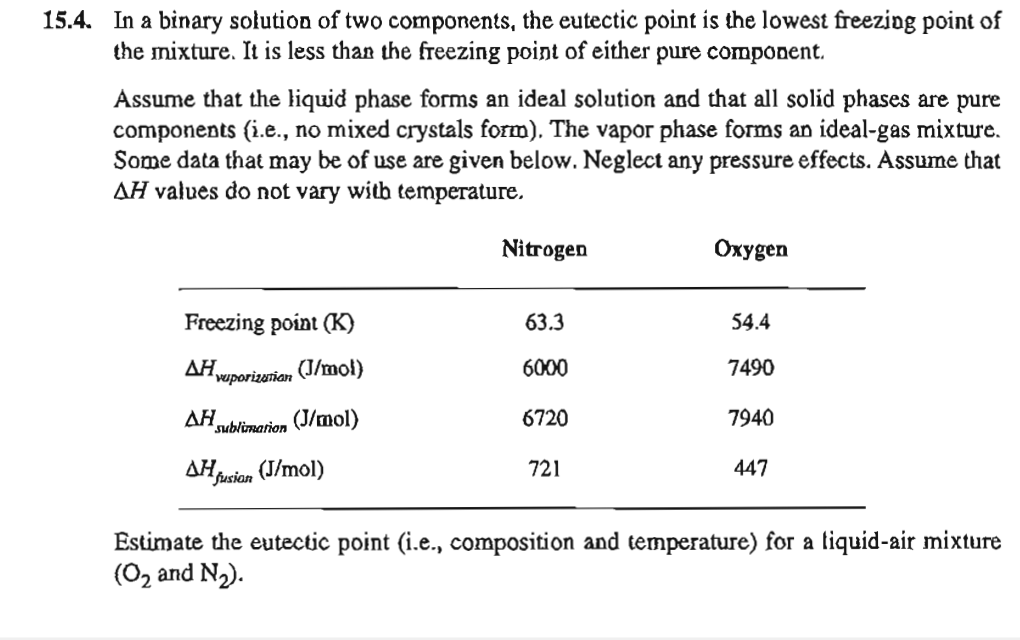
1 5 . 4 . In a binary solution of two components, the eutectic point is the lowest freezing point of the mixture. It is
In a binary solution of two components, the eutectic point is the lowest freezing point of
the mixture. It is less than the freezing point of either pure component.
Assume that the liquid phase forms an ideal solution and that all solid phases are pure
components ie no mixed crystals form The vapor phase forms an idealgas mixture.
Some data that may be of use are given below. Neglect any pressure effects. Assume that
values do not vary witb temperature.
Estimate the eutectic point ie composition and temperature for a liquidair mixture
and :
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


