Answered step by step
Verified Expert Solution
Question
1 Approved Answer
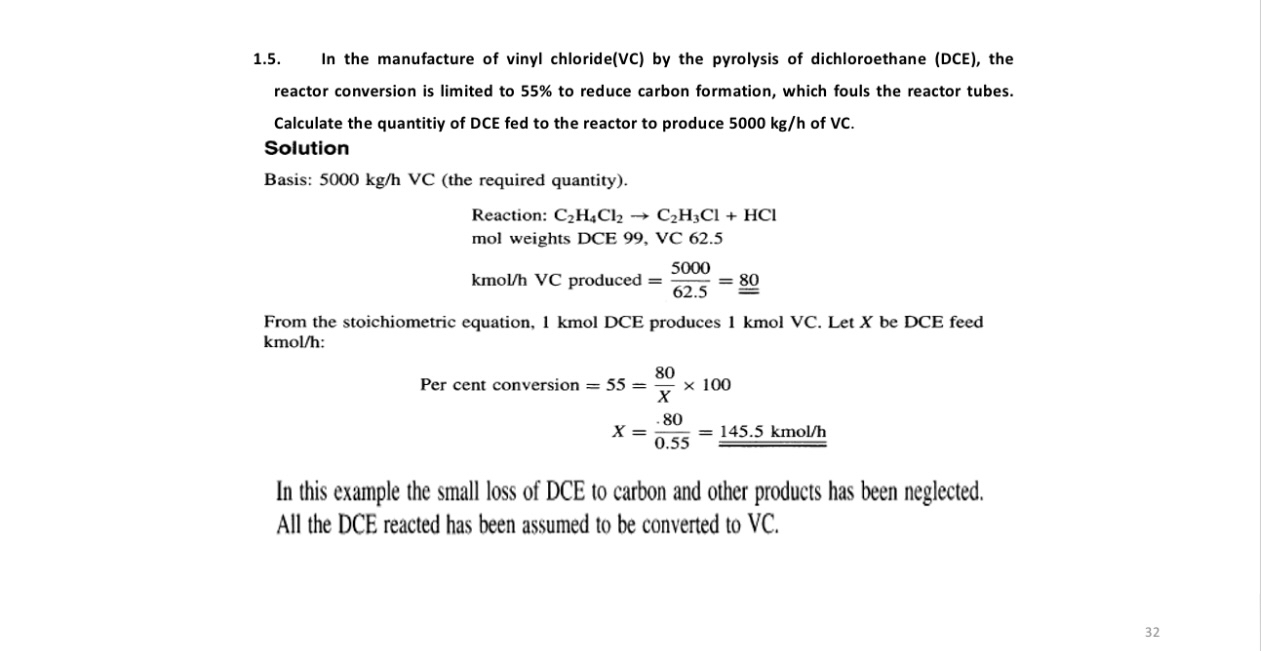
1 . 5 . In the manufacture of vinyl chloride ( VC ) by the pyrolysis of dichloroethane ( DCE ) , the reactor conversion
In the manufacture of vinyl chlorideVC by the pyrolysis of dichloroethane DCE the reactor conversion is limited to to reduce carbon formation, which fouls the reactor tubes.
Calculate the quantitiy of DCE fed to the reactor to produce of VC
Solution
Basis: VC the required quantity
Reaction:
mol weights DCE
kmo produced
From the stoichiometric equation, kmol DCE produces kmol VC Let be DCE feed kmo :
Per cent conversion
kmo
In this example the small loss of DCE to carbon and other products has been neglected. All the DCE reacted has been assumed to be converted to VC
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


