Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. (50 points total) A gas mixture of ammonia (A) and air (B) with the known concentration of Cao flows through a pipe. A long

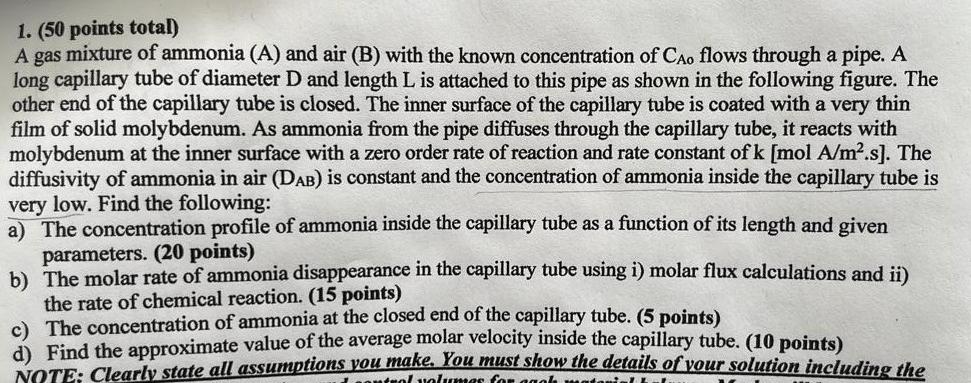
1. (50 points total) A gas mixture of ammonia (A) and air (B) with the known concentration of Cao flows through a pipe. A long capillary tube of diameter and length L is attached to this pipe as shown in the following figure. The other end of the capillary tube is closed. The inner surface of the capillary tube is coated with a very thin film of solid molybdenum. As ammonia from the pipe diffuses through the capillary tube, it reacts with molybdenum at the inner surface with a zero order rate of reaction and rate constant of k [mol A/ms). The diffusivity of ammonia in air (Dau) is constant and the concentration of ammonia inside the capillary tube is very low. Find the following: a) The concentration profile of ammonia inside the capillary tube as a function of its length and given parameters. (20 points) b) The molar rate of ammonia disappearance in the capillary tube usingi) molar flux calculations and i) the rate of chemical reaction. (15 points) c) The concentration of ammonia at the closed end of the capillary tube. (5 points) d) Find the approximate value of the average molar velocity inside the capillary tube (10 points) NOTE: Clearly state all assumptions you make. You must show the details of your solution including the schematic diagram of the system and control volumes for each material balance. Marks will be deducted if the solution steps are not shown. Please separate the answer for each part by drawing a solid line. Air + NH capillary tube 1. (50 points total) A gas mixture of ammonia (A) and air (B) with the known concentration of Cao flows through a pipe. A long capillary tube of diameter D and length L is attached to this pipe as shown in the following figure. The other end of the capillary tube is closed. The inner surface of the capillary tube is coated with a very thin film of solid molybdenum. As ammonia from the pipe diffuses through the capillary tube, it reacts with molybdenum at the inner surface with a zero order rate of reaction and rate constant of k [mol A/m.s]. The diffusivity of ammonia in air (DAB) is constant and the concentration of ammonia inside the capillary tube is very low. Find the following: a) The concentration profile of ammonia inside the capillary tube as a function of its length and given parameters. (20 points) b) The molar rate of ammonia disappearance in the capillary tube using i) molar flux calculations and ii) the rate of chemical reaction. (15 points) c) The concentration of ammonia at the closed end of the capillary tube. (5 points) 1) Find the approximate value of the average molar velocity inside the capillary tube. (10 points) NOTE: Clearly state all assumptions you make. You must show the details of vour solution including the
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


