Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. 98 grams of c alcium chloride (CaCl 2 ) are dissolved in water to prepare one liter of solution. Find normality , molality and
1. 98 grams of calcium chloride (CaCl2) are dissolved in water to prepare one liter of solution. Find normality, molalityand molarity of solution 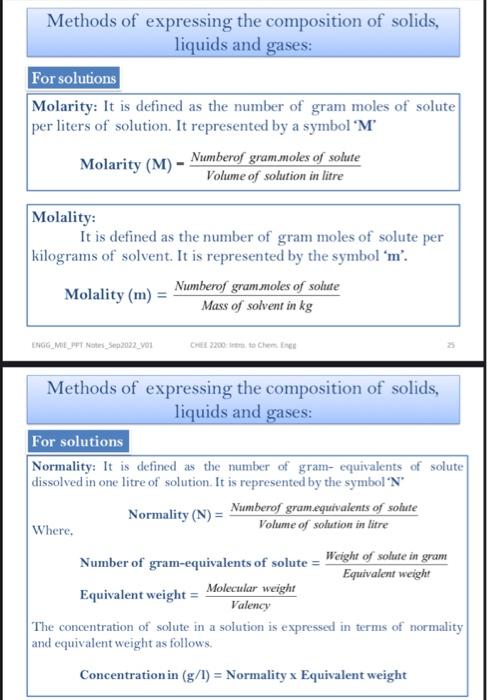 by using this Methods Plz
by using this Methods Plz
Methods of expressing the composition of solids, liquids and gases: Molarity: It is defined as the number of gram moles of solute per liters of solution. It represented by a symbol ' M ' Molarity(M)=VolumeofsolutioninlitreNumberofgram.molesofsolute Molality: It is defined as the number of gram moles of solute per kilograms of solvent. It is represented by the symbol ' m '. Molality(m)=MassofsolventinkgNumberofgrammolesofsolute 8 Methods of expressing the composition of solids, liquids and gases: Normality: It is defined as the number of gram- equivalents of solute dissolved in one litre of solution. It is represented by the symbol " N Where, Normality (N)=VolumeofsolutioninlitreNumberofgram.equivalentsofsolute Numberofgram-equivalentsofsolute=EquivalentweightWeightofsoluteingramEquivalentweight=ValencyMolecularweight The concentration of solute in a solution is expressed in terms of normality and equivalent weight as follows  by using this Methods Plz
by using this Methods PlzStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


