Question
1. A 519-g empty iron kettle is put on a stove. How much heat, in joules, must it absorb to raise its temperature from 13.0C
1.
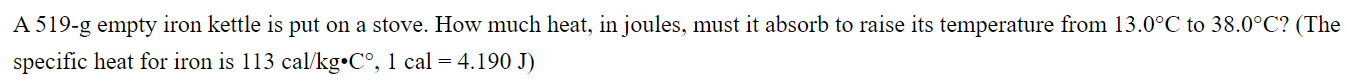



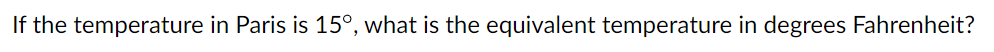
A 519-g empty iron kettle is put on a stove. How much heat, in joules, must it absorb to raise its temperature from 13.0C to 38.0C? (The specific heat for iron is 113 cal/kg C, 1 cal = 4.190 J)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics of Everyday Phenomena A conceptual Introduction to physics
Authors: W. Thomas Griffith, Juliet W. Brosing
6th edition
9780073513904, 73513903, 9781259894008, 1259894002, 978-0073512112
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



