Question
1.- A cell is set up as follows: Zn (s) /Zn 2+ (1M)//Ag + (1M)/Ag (s) E 0 (Zn 2+ /Zn)=-0.76 V ; E 0
1.- A cell is set up as follows:
Zn(s)/Zn2+(1M)//Ag+(1M)/Ag(s)
E0(Zn2+/Zn)=-0.76 V ; E0 (Ag+/Ag) = 0.80 V
i) Write the cell reaction ii) Calculate the cell voltage iii) Determine K at equilibrium
2.- According to the concept of hard and soft acid-base, what happens in the following reaction:
ZnI2+HgCl2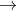 ZnCl2+HgI2
ZnCl2+HgI2
3.- Determine the appropriate enthalpy change according to the Lux-Flood Theory for the following reaction:
a) SO3+H2OH2SO4
4.- Use the Valence Bond Theory (VTE) for the following molecules: a) CCl4 b) Ni(CO)4
5.- Solve the following Latimer diagrams:
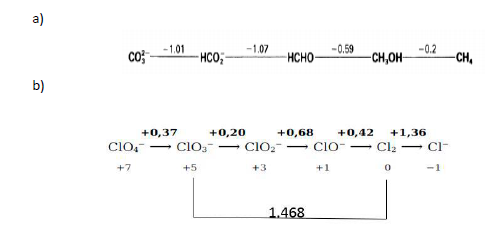
1 a) - 1.01 -1.07 -0.59 -0.2 CO -HCO, H- -CH,OH -CH, b) +0,37 +0,20 +0,68 +0,42 +1,36 CIO, - CIO CO2- =C10-Cl2 Cl- +7 +5 +3 +1 0 -1 1.468
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


