Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. a. Excited lithium atoms emit red light of wavelength 6.71 x 10-5cm. (i) Determine the frequency, v, in s', of this radiation () Calculate
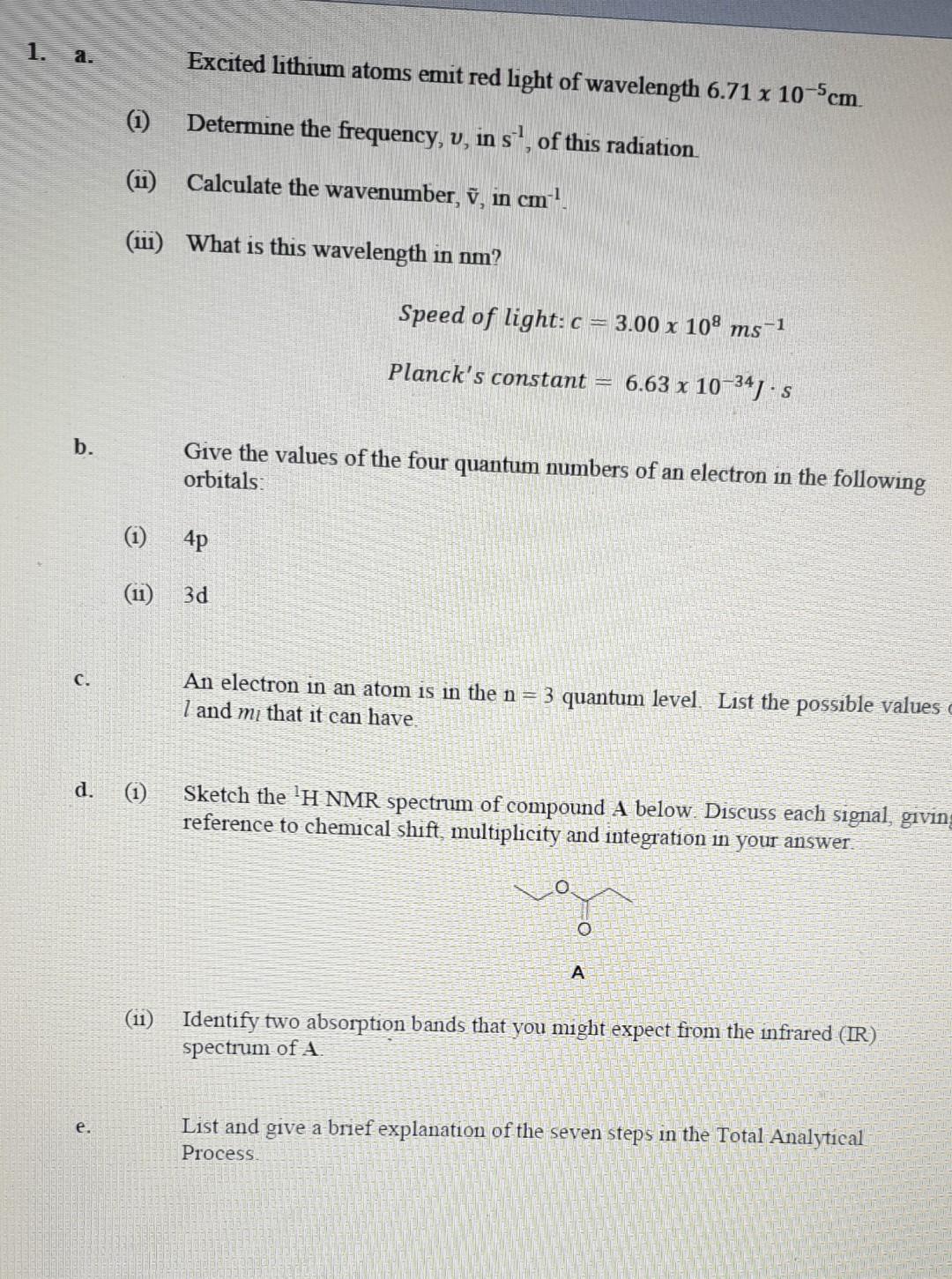
1. a. Excited lithium atoms emit red light of wavelength 6.71 x 10-5cm. (i) Determine the frequency, v, in s', of this radiation () Calculate the wavenumber, , in cm (w) What is this wavelength in nm? Speed of light: c = 3.00 x 108 ms -1 Planck's constant = 6.63 x 10-34) s b. Give the values of the four quantum numbers of an electron in the following orbitals: (1) 4p (1) 3d C. An electron in an atom is in the n = 3 quantum level. List the possible values I and m that it can have. d. (1) Sketch the H NMR spectrum of compound A below. Discuss each signal, giving reference to chemical shift, multiplicity and integration in your answer A (11) Identify two absorption bands that you might expect from the infrared (IR) spectrum of A List and give a brief explanation of the seven steps in the Total Analytical Process
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


