Question
1. A flask has a mass of 78.23 g when empty and 593.63 g when filled with water. When the same flask is filled
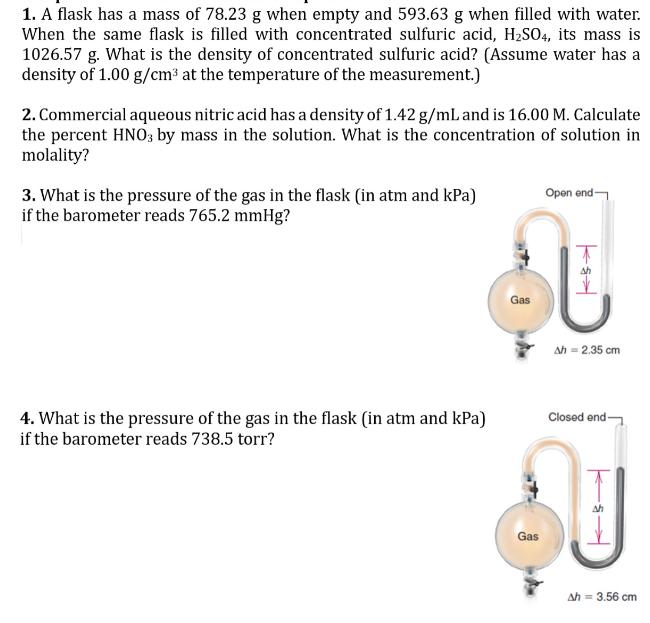
1. A flask has a mass of 78.23 g when empty and 593.63 g when filled with water. When the same flask is filled with concentrated sulfuric acid, H2SO4, its mass is 1026.57 g. What is the density of concentrated sulfuric acid? (Assume water has a density of 1.00 g/cm at the temperature of the measurement.) 2. Commercial aqueous nitric acid has a density of 1.42 g/mL and is 16.00 M. Calculate the percent HNO3 by mass in the solution. What is the concentration of solution in molality? 3. What is the pressure of the gas in the flask (in atm and kPa) if the barometer reads 765.2 mmHg? Open end- 4. What is the pressure of the gas in the flask (in atm and kPa) if the barometer reads 738.5 torr? Gas Gas - T Ah = 2.35 cm Closed end- Ah = 3.56 cm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: James S. Walker
5th edition
978-0133498493, 9780321909107, 133498492, 0321909100, 978-0321976444
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



