Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1 A local FM radio station broadcasts at a frequency of 88.5 MHz. Calculate the wavelength at which it is broadcasting, Wavelength meter (1 MHz


1
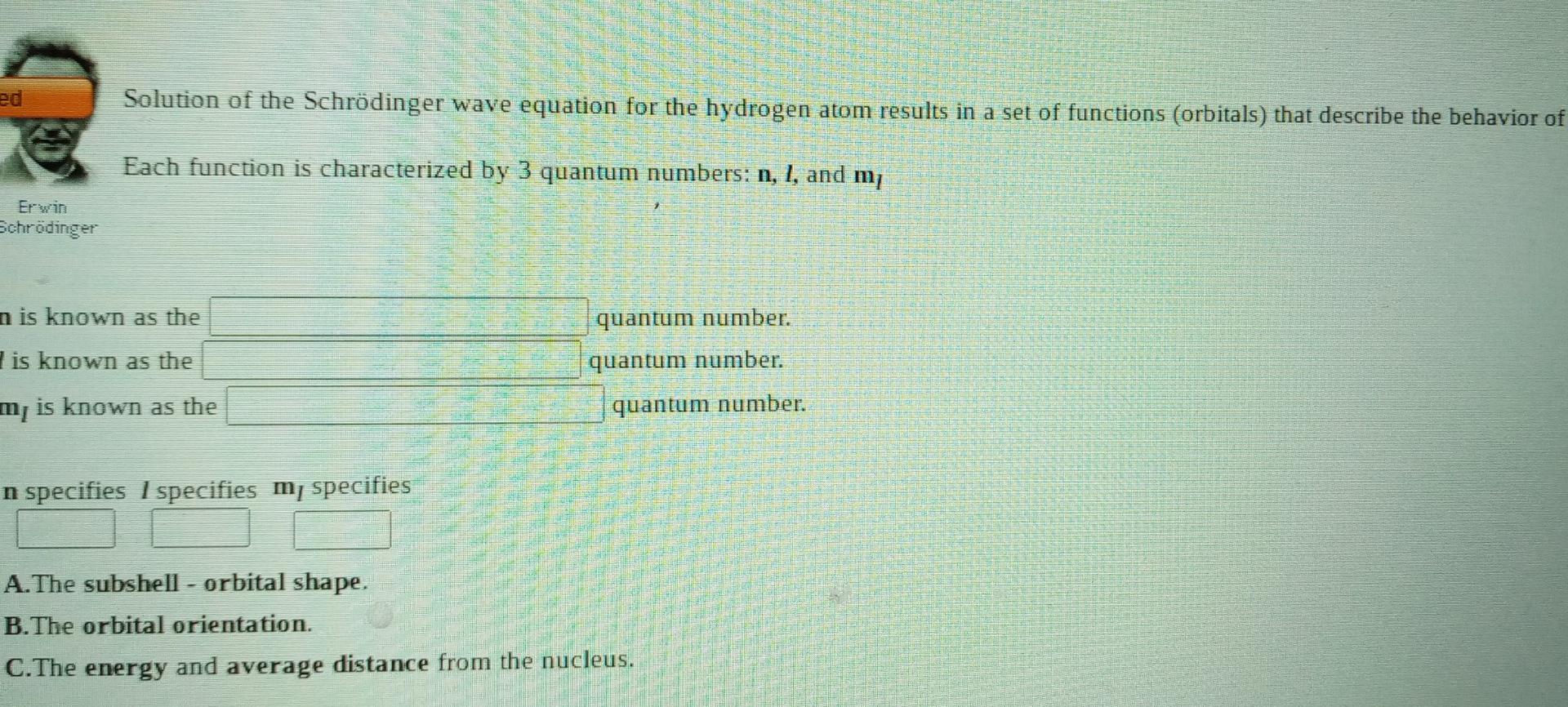
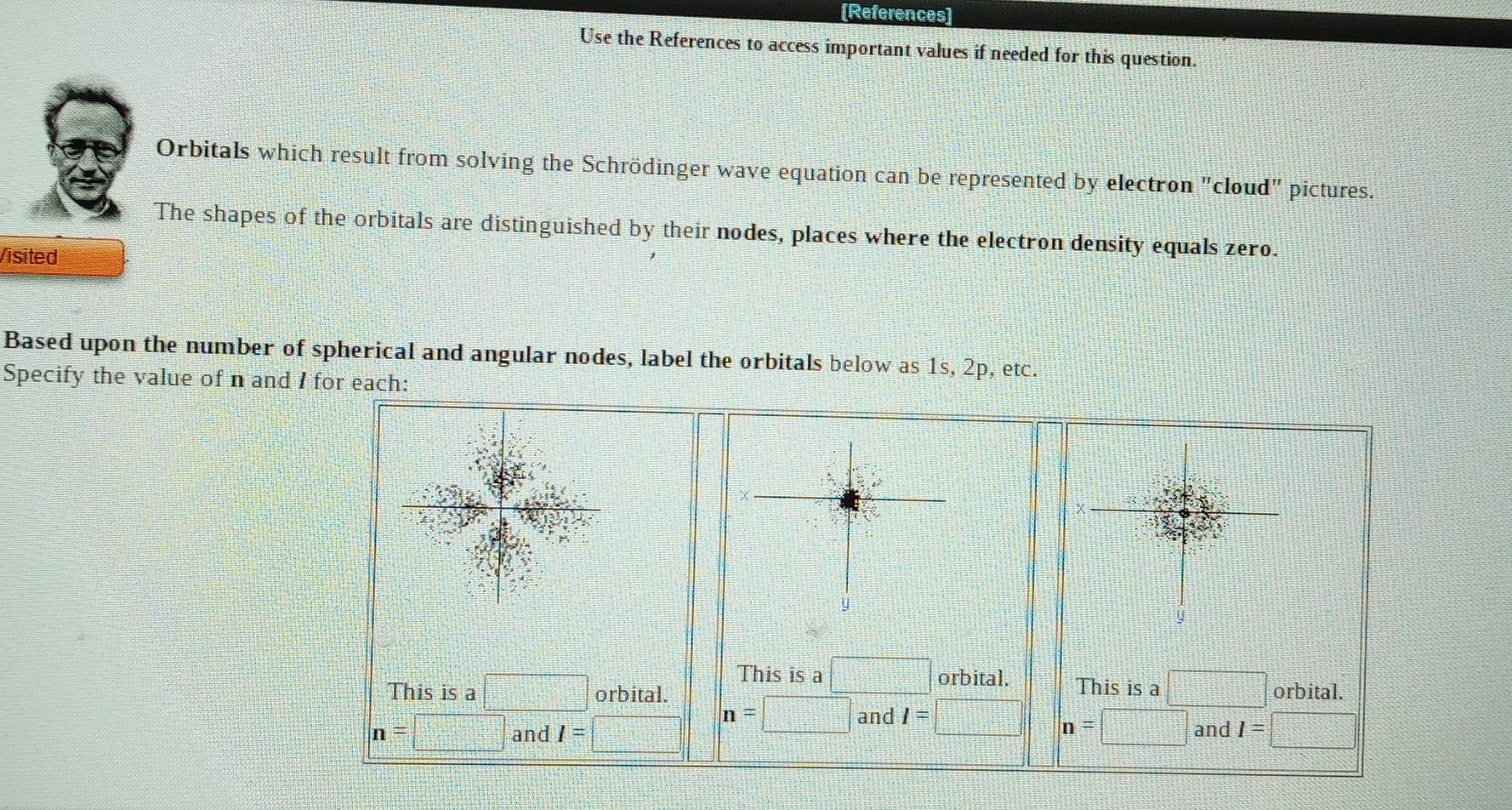
A local FM radio station broadcasts at a frequency of 88.5 MHz. Calculate the wavelength at which it is broadcasting, Wavelength meter (1 MHz = 10s-1) Submit Answer Try Another Version 10 item attempts remaining ed Solution of the Schrdinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of Each function is characterized by 3 quantum numbers: n, 1, and my Erwin Schrdinger n is known as the quantum number. quantum number. I is known as the mis known as the quantum number. n specifies I specifies my specifies A. The subshell - orbital shape. B.The orbital orientation. C.The energy and average distance from the nucleus. (References) Use the References to access important values if needed for this question. Orbitals which result from solving the Schrdinger wave equation can be represented by electron "cloud" pictures. The shapes of the orbitals are distinguished by their nodes, places where the electron density equals zero. Jisited Based upon the number of spherical and angular no des, label the orbitals below as 1s, 2p, etc. Specify the value of n and I for each: This is a orbital. This is a orbital. This is a orbital. n- and 1 = n and 1 = n = and 1 = The Pauli Exclusion Principle tells us that no two electrons in an atom can have the same four quantum numbers. For an electron in the 2p orbital shown above, enter a possible value for each quantum number. Give ONE example. Give ONE example. possible m, values for electrons in 2p orbitals. Though a given electron only has one value for mi, there areStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


