Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. A sample of runoff from a farm was collected and analyzed for its iron content. A 25.0ml sample was acidified with nitric acid and
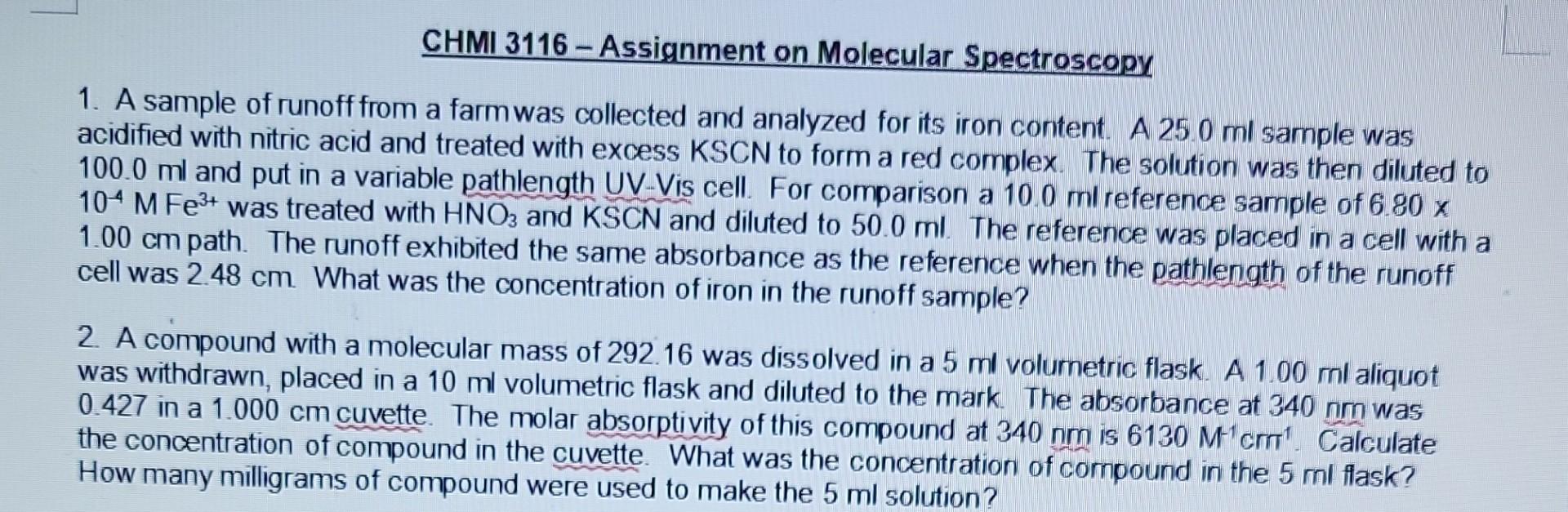
1. A sample of runoff from a farm was collected and analyzed for its iron content. A 25.0ml sample was acidified with nitric acid and treated with excess KSCN to form a red complex. The solution was then diluted to 100.0ml and put in a variable pathlength UV-Vis cell. For comparison a 10.0ml reference sample of 6.80x 104MFe3+ was treated with HNO3 and KSCN and diluted to 50.0ml. The reference was placed in a cell with a 1.00cm path. The runoff exhibited the same absorbance as the reference when the pathlength of the runoff cell was 2.48cm. What was the concentration of iron in the runoff sample? 2. A compound with a molecular mass of 292.16 was dissolved in a 5ml volumetric flask. A 1.00ml aliquot was withdrawn, placed in a 10ml volumetric flask and diluted to the mark. The absorbance at 340nm was 0.427 in a 1.000cm cuvette. The molar absorptivity of this compound at 340nm is 6130M1cm1. Calculate the concentration of compound in the cuvette. What was the concentration of compound in the 5ml flask? How many milligrams of compound were used to make the 5ml solution
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


