Question
1) A student measured the melting point of Compound X and compared it to a list of possible known compounds. The student narrowed down the
1) A student measured the melting point of Compound X and compared it to a list of possible known compounds. The student narrowed down the list of possibilities to Compounds A, B, and C (all of which had similar melting points). The student developed a TLC plate using an appropriate eluant, in order to observe the relative Rf s of each of the compounds. The outcome is illustrated below:
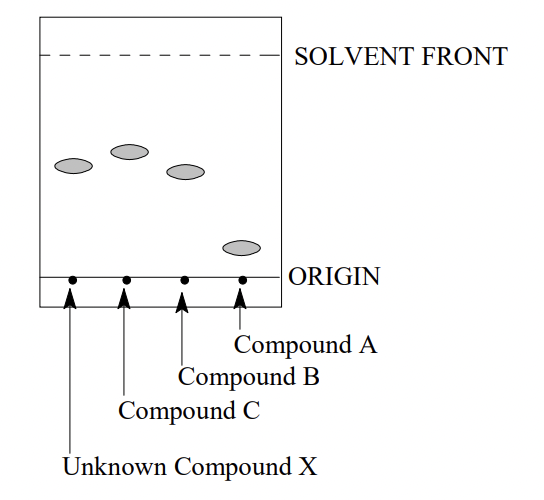
Which of the following statements is most true:
1. Compound C appears to be less polar than Compound X
2. Compound A is not the same as Compound X
3. Rf values provides supporting evidence that Compound B might be Compound X
4. Compound A is more polar than compound X
5. All of the above are true.
2.A student produced the following TLC using Hexane: Ethyl acetate (90:10) as the mobile phase
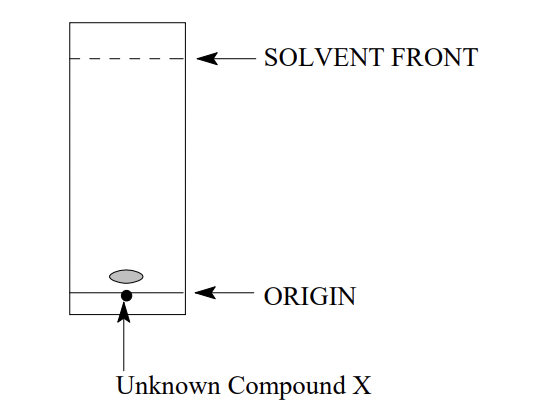
To repeat the experiment and get a higher Rf value, the student should
a) spot more sample on the TLC plate
b) use hexane as the mobile phase
c) use Hexane: Ethyl acetate (70:30) as the mobile phase
d) use a longer time to allow the solvent to migrate further up the plate.
SOLVENT FRONT ORIGIN Compound A Compound B Compound C Unknown Compound X SOLVENT FRONT ORIGIN Unknown Compound XStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


