Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1 (a) Two reagents that can be used to prepare chlorine gas are manganese (IV) oxide and concentrated hydrochloric acid. Write an equation for
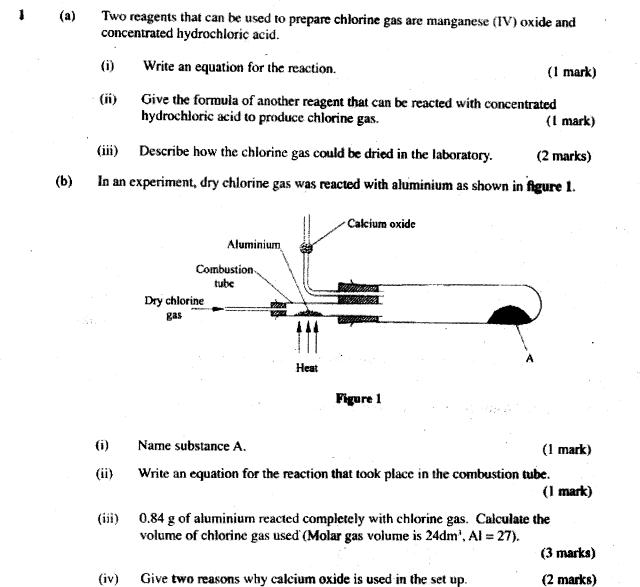
1 (a) Two reagents that can be used to prepare chlorine gas are manganese (IV) oxide and concentrated hydrochloric acid. Write an equation for the reaction. Give the formula of another reagent that can be reacted with concentrated hydrochloric acid to produce chlorine gas. (1 mark) (iii) Describe how the chlorine gas could be dried in the laboratory. (2 marks) (b) In an experiment, dry chlorine gas was reacted with aluminium as shown in figure 1. (i) (ii) (iv) Aluminium Combustion tube Dry chlorine gas Heat Calcium oxide Figure 1 (iii) 0.84 g of aluminium reacted completely with chlorine gas. Calculate volume of chlorine gas used' (Molar gas volume is 24dm, Al = 27). Name substance A. (1 mark) Write an equation for the reaction that took place in the combustion tube. (1 mark) (1 mark) Give two reasons why calcium oxide is used in the set up. the (3 marks) (2 marks)
Step by Step Solution
★★★★★
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
a Two reagents that can be used to prepare chlorine gas are manganese IV oxide and concentrated HCL I Write the equation for the reaction taking place ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


