Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. A zinc/bromine secondary battery involves the following electrochemical half-reactions in an aqueous solution of zinc bromide: Zn2+ + 2eZn E = -0.76 V
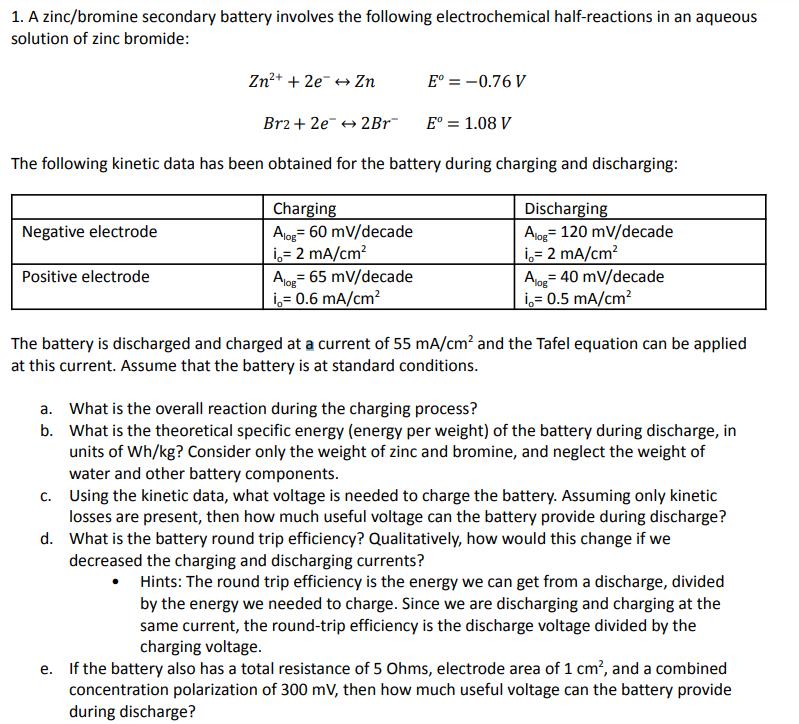
1. A zinc/bromine secondary battery involves the following electrochemical half-reactions in an aqueous solution of zinc bromide: Zn2+ + 2eZn E = -0.76 V E = 1.08 V Br2+2e2Br The following kinetic data has been obtained for the battery during charging and discharging: Negative electrode Positive electrode Charging Alog 60 mV/decade i = 2 mA/cm = Alog 65 mV/decade i= 0.6 mA/cm Discharging Alog 120 mV/decade i = 2 mA/cm Alo 40 mV/decade i= 0.5 mA/cm The battery is discharged and charged at a current of 55 mA/cm and the Tafel equation can be applied at this current. Assume that the battery is at standard conditions. a. What is the overall reaction during the charging process? b. What is the theoretical specific energy (energy per weight) of the battery during discharge, in units of Wh/kg? Consider only the weight of zinc and bromine, and neglect the weight of water and other battery components. c. Using the kinetic data, what voltage is needed to charge the battery. Assuming only kinetic losses are present, then how much useful voltage can the battery provide during discharge? d. What is the battery round trip efficiency? Qualitatively, how would this change if we decreased the charging and discharging currents? Hints: The round trip efficiency is the energy we can get from a discharge, divided by the energy we needed to charge. Since we are discharging and charging at the same current, the round-trip efficiency is the discharge voltage divided by the charging voltage. e. If the battery also has a total resistance of 5 Ohms, electrode area of 1 cm, and a combined concentration polarization of 300 mV, then how much useful voltage can the battery provide during discharge?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


