Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Answer BOTH parts. (a) In the presence of the enzyme lactate dehydrogenase, pyruvate is reduced by nicotinamide adenine dinucleotide (NAD) according to the equation:
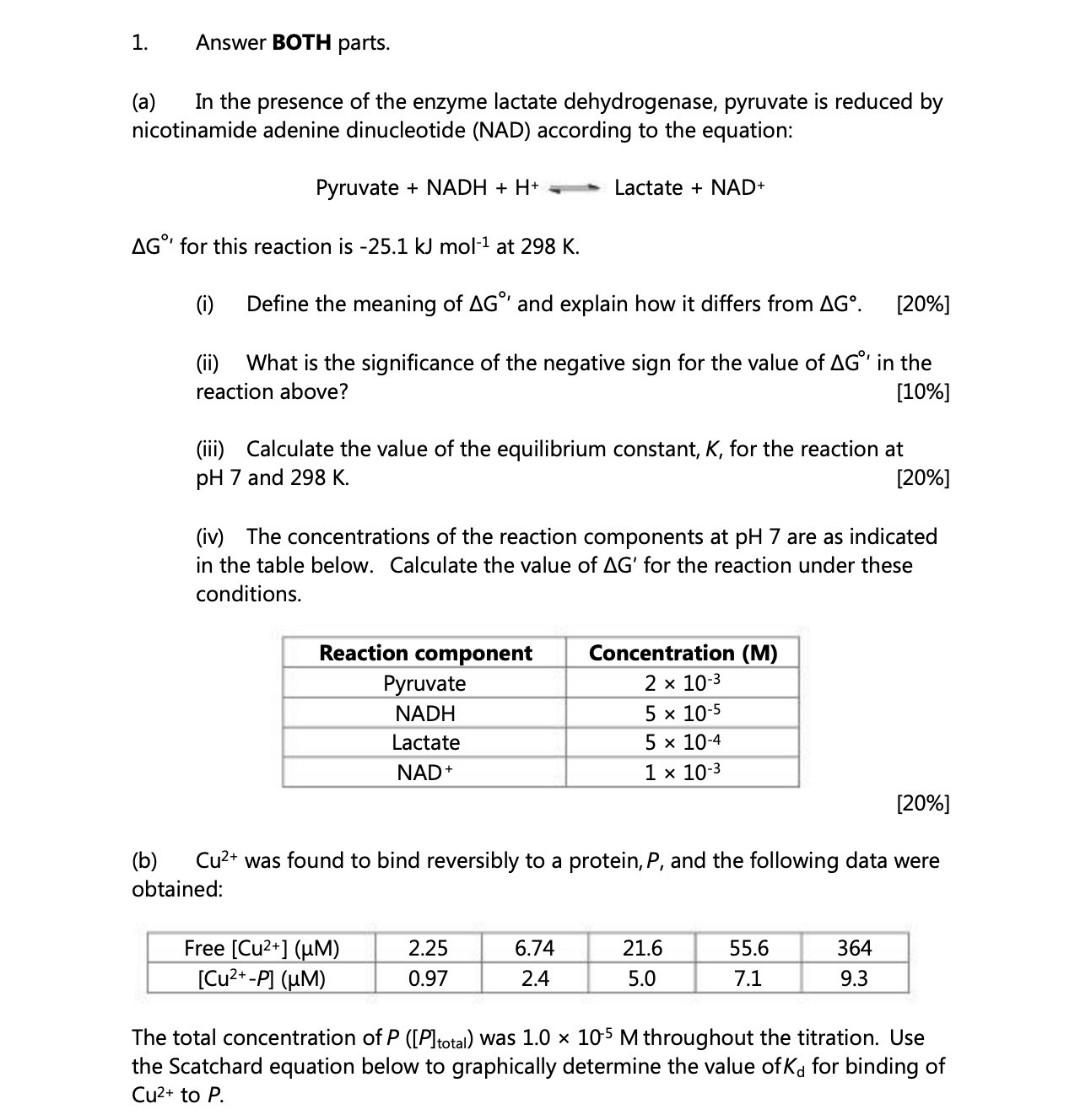
1. Answer BOTH parts. (a) In the presence of the enzyme lactate dehydrogenase, pyruvate is reduced by nicotinamide adenine dinucleotide (NAD) according to the equation: Pyruvate+NADH+H+Lactate+NAD+ GI for this reaction is 25.1kJmol1 at 298K. (i) Define the meaning of GI and explain how it differs from G. [20\%] (ii) What is the significance of the negative sign for the value of G in the reaction above? [10\%] (iii) Calculate the value of the equilibrium constant, K, for the reaction at pH 7 and 298K. [20\%] (iv) The concentrations of the reaction components at pH7 are as indicated in the table below. Calculate the value of G for the reaction under these conditions. [20\%] (b) Cu2+ was found to bind reversibly to a protein, P, and the following data were obtained: The total concentration of P([P]total) was 1.0105M throughout the titration. Use the Scatchard equation below to graphically determine the value of Kd for binding of Cu2+ to P
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


