Answered step by step
Verified Expert Solution
Question
1 Approved Answer
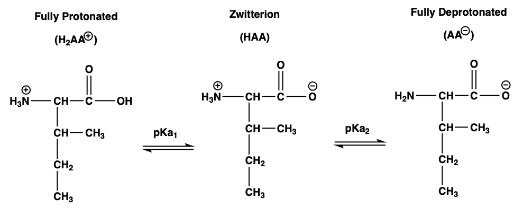
1) At which pH will a solution of isoleucine be fully in it's zwitterion species, 100% = [HAA] pKa1 = 2.36 pKa2 = 9.68 pI
1) At which pH will a solution of isoleucine be fully in it's zwitterion species, 100% = [HAA]
pKa1 = 2.36
pKa2 = 9.68
pI = 6.02
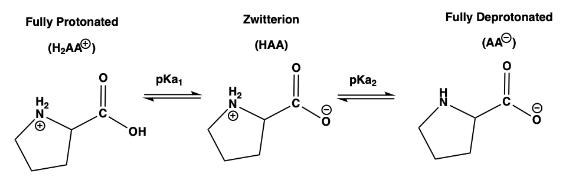
2) At which pH will a solution of proline have equal concentrations of its fully protonated species and zwitterion species, [H2AA+] = [HAA
pKa1 = 1.99
pKa2 = 10.96
pI = 6.48
Fully Protonated Zwitterion Fully Deprotonated (H2AA) (HAA) (AA) pKa1 =pKa2 Fully Protonated Zwitterion Fully Deprotonated (H2AA) (HAA) (AA) pKa1 pKa2
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


