Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(1) Calculate the equilibrium constant for the following reaction: FeO(s)+CO(g)Fe(s)+CO2(g) if at 298K the equilibrium amounts present are 2.5molFeO,0.2molFe,3.0mol CO2 and 4.0molCO. (2) For the
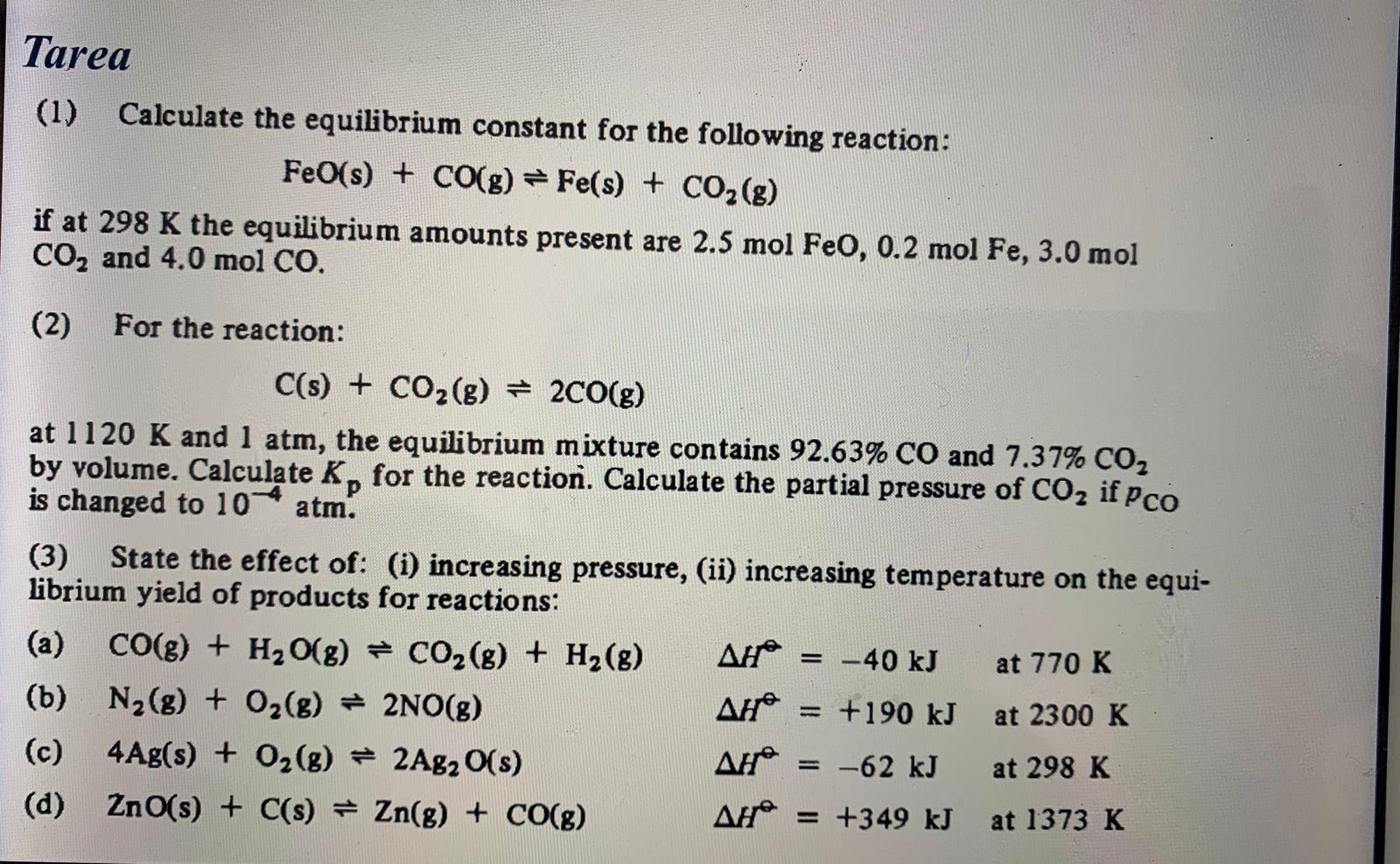
(1) Calculate the equilibrium constant for the following reaction: FeO(s)+CO(g)Fe(s)+CO2(g) if at 298K the equilibrium amounts present are 2.5molFeO,0.2molFe,3.0mol CO2 and 4.0molCO. (2) For the reaction: C(s)+CO2(g)2CO(g) at 1120K and 1atm, the equilibrium mixture contains 92.63%CO and 7.37%CO2 by volume. Calculate Kp for the reaction. Calculate the partial pressure of CO2 if pCO is changed to 104atm. (3) State the effect of: (i) increasing pressure, (ii) increasing temperature on the equilibrium yield of products for reactions: (a) CO(g)+H2O(g)CO2(g)+H2(g)H=40kJ at 770K (b) N2(g)+O2(g)2NO(g)H=+190kJ at 2300K (c) 4Ag(s)+O2(g)2Ag2O(s)H=62kJ at 298K (d) ZnO(s)+C(s)Zn(g)+CO(g)H=+349kJ at 1373K
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


