Question
The elementary step reversible reaction shown below occurs in the liquid phase in a plug flow reactor. The feed contains only A at a
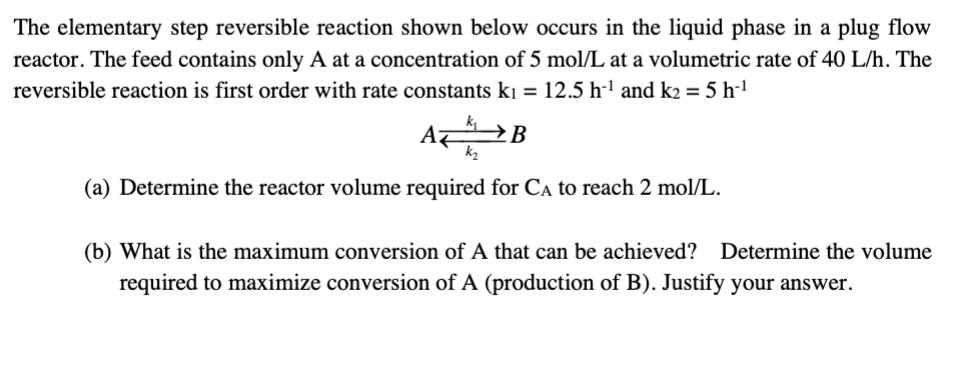
The elementary step reversible reaction shown below occurs in the liquid phase in a plug flow reactor. The feed contains only A at a concentration of 5 mol/L at a volumetric rate of 40 L/h. The reversible reaction is first order with rate constants k = 12.5 h and k2=5h-1 AB (a) Determine the reactor volume required for CA to reach 2 mol/L. (b) What is the maximum conversion of A that can be achieved? Determine the volume required to maximize conversion of A (production of B). Justify your answer.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a Given Rate equation for forward reaction Rf k1CA Rate equation for backward reaction Rb k2CB ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Accounting Tools for Business Decision Making
Authors: Paul D. Kimmel, Jerry J. Weygandt, Donald E. Kieso
9th edition
1-119-49356-3, 1119493633, 1119493560, 978-1119493631
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



