Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Calculate the probability of an electron in the 3dz2 orbital being found in the donut. The 3dz2 orbital is given by: 8161(a0Z)3/22e/3(3cos21) where =Zr/a0.
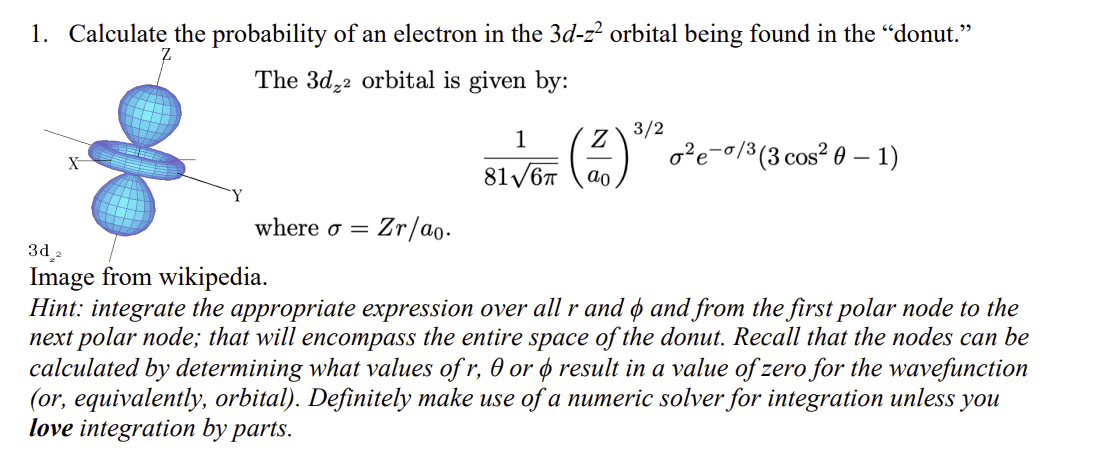 1. Calculate the probability of an electron in the 3dz2 orbital being found in the "donut." The 3dz2 orbital is given by: 8161(a0Z)3/22e/3(3cos21) where =Zr/a0. Image from wikipedia. Hint: integrate the appropriate expression over all r and and from the first polar node to the next polar node; that will encompass the entire space of the donut. Recall that the nodes can be calculated by determining what values of r, or result in a value of zero for the wavefunction (or, equivalently, orbital). Definitely make use of a numeric solver for integration unless you love integration by parts
1. Calculate the probability of an electron in the 3dz2 orbital being found in the "donut." The 3dz2 orbital is given by: 8161(a0Z)3/22e/3(3cos21) where =Zr/a0. Image from wikipedia. Hint: integrate the appropriate expression over all r and and from the first polar node to the next polar node; that will encompass the entire space of the donut. Recall that the nodes can be calculated by determining what values of r, or result in a value of zero for the wavefunction (or, equivalently, orbital). Definitely make use of a numeric solver for integration unless you love integration by parts Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


