Answered step by step
Verified Expert Solution
Question
1 Approved Answer
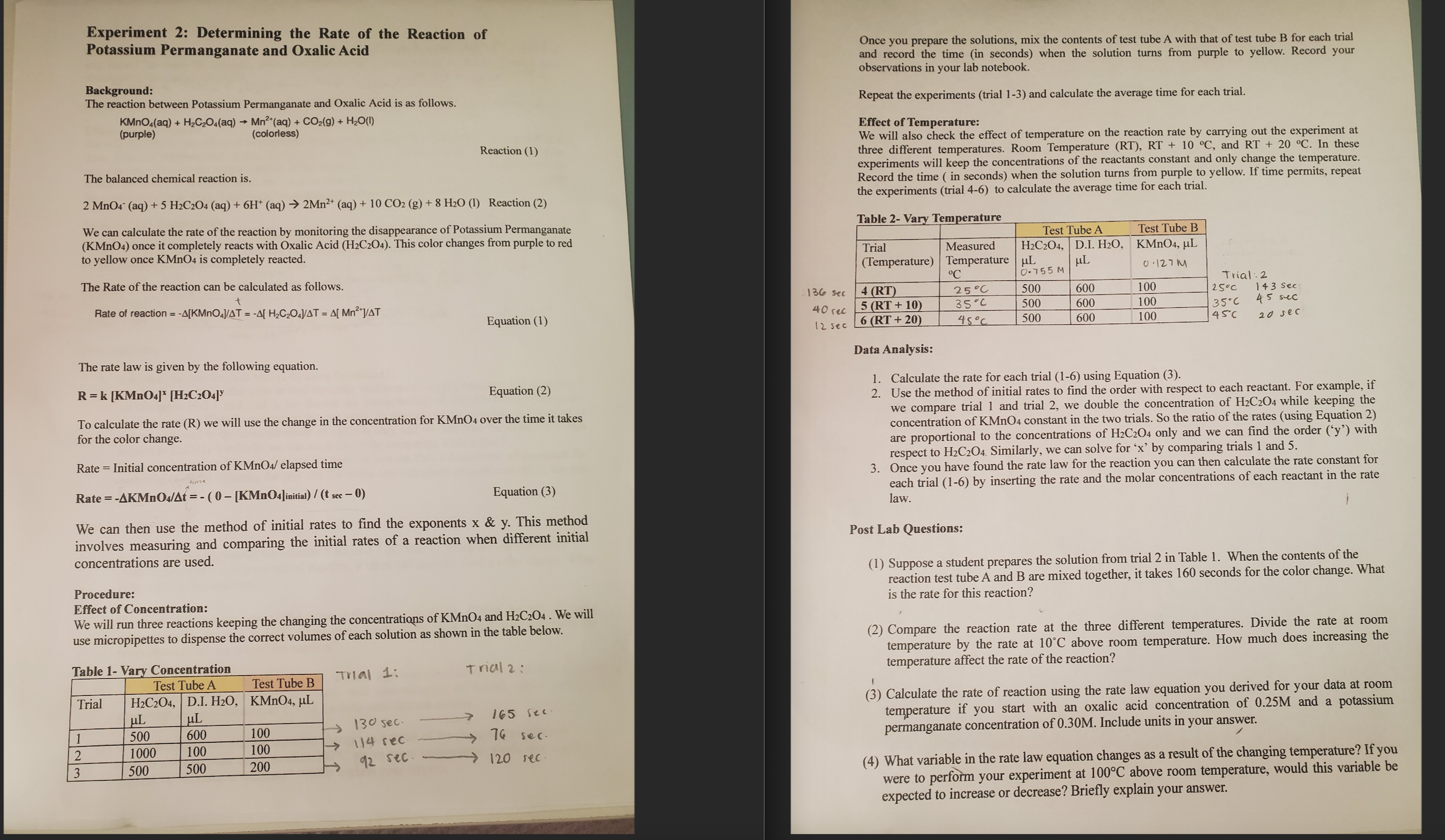
1 . Calculate the rate for each trial ( 1 - 6 ) using Equation ( 3 ) . 2 . Use the method of
Calculate the rate for each trial using Equation
Use the method of initial rates to find the order with respect to each reactant. For example, if we compare trial and trial we double the concentration of HCO while keeping the concentration of KMnO constant in the two trials. So the ratio of the rates using Equation are proportional to the concentrations of HCO only and we can find the order y with respect to HCO Similarly, we can solve for x by comparing trials and
Once you have found the rate law for the reaction you can then calculate the rate constant for each trial by inserting the rate and the molar concentrations of each reactant in the rate law.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


