Question
1. Calculate the theoretical (thermodynamic) specific energy of the hydrazine fuel cell expressed in [kWh / kg_hydrazine] and compare it with the H2 fuel cell.
1. Calculate the theoretical (thermodynamic) specific energy of the hydrazine fuel cell expressed in [kWh / kg_hydrazine] and compare it with the H2 fuel cell. Note: if you require thermodynamic data use Handbooks available on-line through UBC Library. B) If the fuel cell is operated with a superficial current density of 500 mA/cm2 and the electrode geometric area is 250 cm2, calculate the following: i) extent of fuel cell reaction [molreaction/s], ii) the volumetric inlet flow rate [cm3/min] for the anolyte solution composed of 4 M hydrazine in 4 M KOH assuming the goal is to have a high hydrazine conversion at the anode of 90%, iii) the volumetric flow rate [cm3/min] of N2 gas produced at 1 atm and 60 oC, iv) the mass of hydrazine [g] consumed during 1 hour of continuous operation. Assume steady-state. C) Calculate the: i) air flow rate [cm3/min, at 273 K and 1 atm, air 20% O2 by vol.] stoichiometrically required to sustain the anode reaction as described at question 2, and ii) the molar flow rate of hydroxide crossing the membrane.
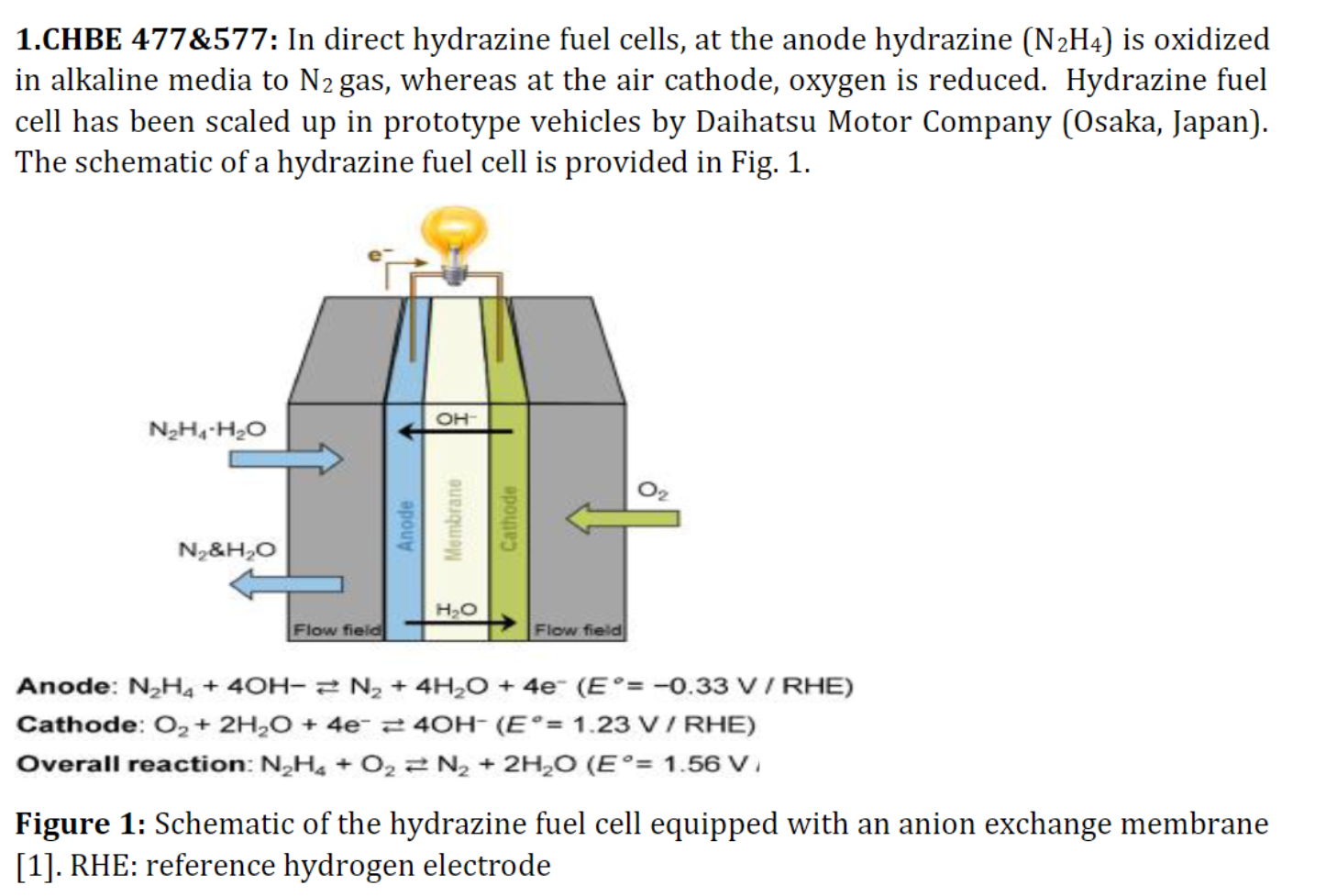
1.CHBE 477\&577: In direct hydrazine fuel cells, at the anode hydrazine (N2H4) is oxidized in alkaline media to N2 gas, whereas at the air cathode, oxygen is reduced. Hydrazine fuel cell has been scaled up in prototype vehicles by Daihatsu Motor Company (Osaka, Japan). The schematic of a hydrazine fuel cell is provided in Fig. 1. Anode: N2H4+4OHN2+4H2O+4e(E=0.33V/RHE) Cathode: O2+2H2O+4e4OH(E=1.23V/RHE) Overall reaction: N2H4+O2N2+2H2O(E=1.56V i Figure 1: Schematic of the hydrazine fuel cell equipped with an anion exchange membrane [1]. RHE: reference hydrogen electrodeStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


