Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Carbon dioxide (CO2) gas undergoes a process in a well-insulated piston-cylinder assembly from P1 = 2 bar, T1 = 300 K to P2

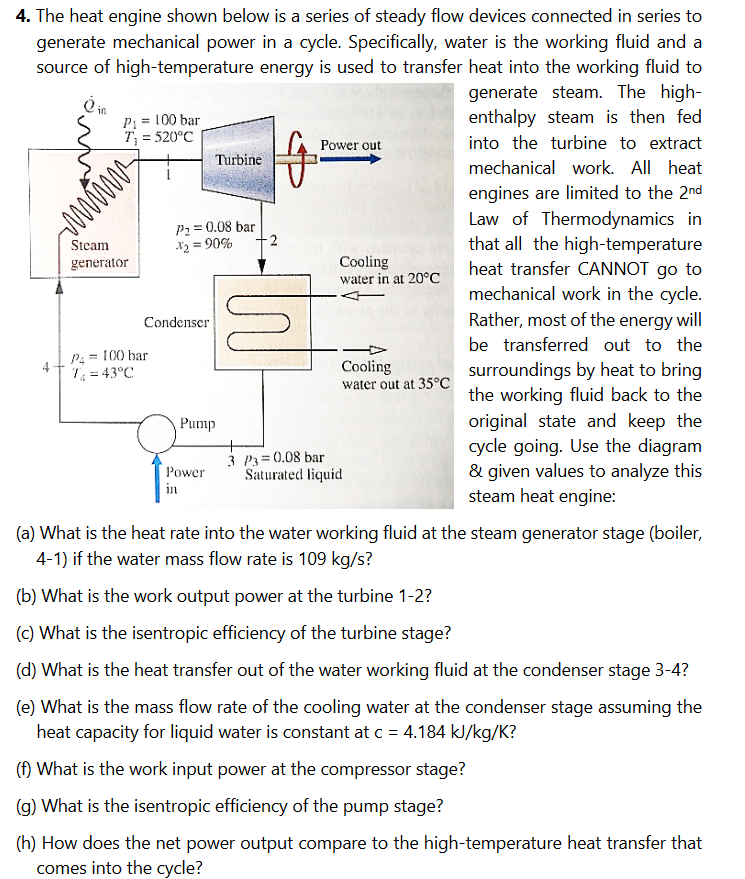
1. Carbon dioxide (CO2) gas undergoes a process in a well-insulated piston-cylinder assembly from P1 = 2 bar, T1 = 300 K to P2 = 20 bar, T2 = 1700 K. Assuming this CO2 behaves like an ideal gas: (a) What is the intensive entropy change using a constant, average value for heat capacity that represents the relevant range of process conditions? (b) What is the intensive entropy change accounting for the change of specific heat with temperature? (c) what is the percent difference between results using the different models? (d) which of these is most accurate, why? (e) for case (b), what is the entropy generation for this process? 2. A steady-flow turbine running on air working fluid has P1 = 8 bar, T1 = 1400 K at the inlet and the air expands to P2 = 0.8 bar at the turbine exit. Assume there is no heat loss, no significant KE and PE effects and accounting for temperature effects of heat capacity. Under these assumptions, what is the theoretical limit for the intensive work involved in this turbine process if the air behaves like an ideal gas? 3. Nitrogen is flowing at steady state through a well-insulated diffuser with inlet conditions of P1 = 0.656 bar, T1 = 300 K and v = 292 m/s. The exit conditions of the nitrogen flow are P2 = 0.9 bar and v2 = 130 m/s. The inlet area is A1 = 4.8 10-3 m. (a) What is the extensive entropy change rate (kJ/K/s) for the process using cold air standard assumptions? (b) What is the area at the exit assuming cold air standard assumptions? 4. The heat engine shown below is a series of steady flow devices connected in series to generate mechanical power in a cycle. Specifically, water is the working fluid and a source of high-temperature energy is used to transfer heat into the working fluid to in P = 100 bar T = 520C www Power out Turbine P = 0.08 bar *2=90% -2 generator Steam 4 P = 100 bar 7 =43C Condenser Cooling water in at 20C Pump 3 P3 0.08 bar Power in Saturated liquid Cooling water out at 35C generate steam. The high- enthalpy steam is then fed into the turbine to extract mechanical work. All heat engines are limited to the 2nd Law of Thermodynamics in that all the high-temperature heat transfer CANNOT go to mechanical work in the cycle. Rather, most of the energy will be transferred out to the surroundings by heat to bring the working fluid back to the original state and keep the cycle going. Use the diagram & given values to analyze this steam heat engine: (a) What is the heat rate into the water working fluid at the steam generator stage (boiler, 4-1) if the water mass flow rate is 109 kg/s? (b) What is the work output power at the turbine 1-2? (c) What is the isentropic efficiency of the turbine stage? (d) What is the heat transfer out of the water working fluid at the condenser stage 3-4? (e) What is the mass flow rate of the cooling water at the condenser stage assuming the heat capacity for liquid water is constant at c = 4.184 kJ/kg/K? (f) What is the work input power at the compressor stage? (g) What is the isentropic efficiency of the pump stage? (h) How does the net power output compare to the high-temperature heat transfer that comes into the cycle?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


