Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Consider a catalyst particle of radius R. This particle is in a catalytic reactor, where it is submerged in a gas stream containing the
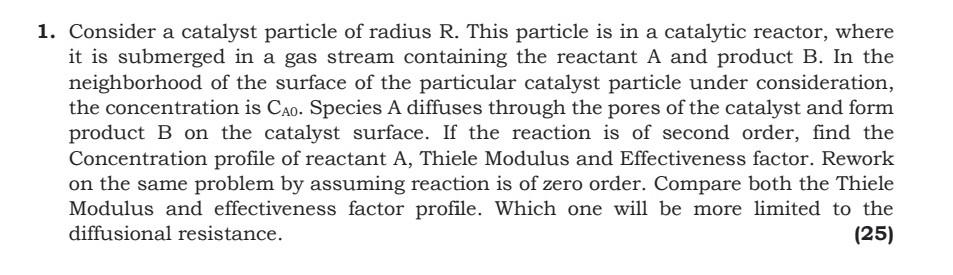
1. Consider a catalyst particle of radius R. This particle is in a catalytic reactor, where it is submerged in a gas stream containing the reactant A and product B. In the neighborhood of the surface of the particular catalyst particle under consideration, the concentration is Cao. Species A diffuses through the pores of the catalyst and form product B on the catalyst surface. If the reaction is of second order, find the Concentration profile of reactant A, Thiele Modulus and Effectiveness factor. Rework on the same problem by assuming reaction is of zero order. Compare both the Thiele Modulus and effectiveness factor profile. Which one will be more limited to the diffusional resistance. (25)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


