Question
1. Consider a fuel cell stack that can operate at either 300kPa or 170kPa (inlet pressure) with oxygen stoichiometric ratio of 5. The stack
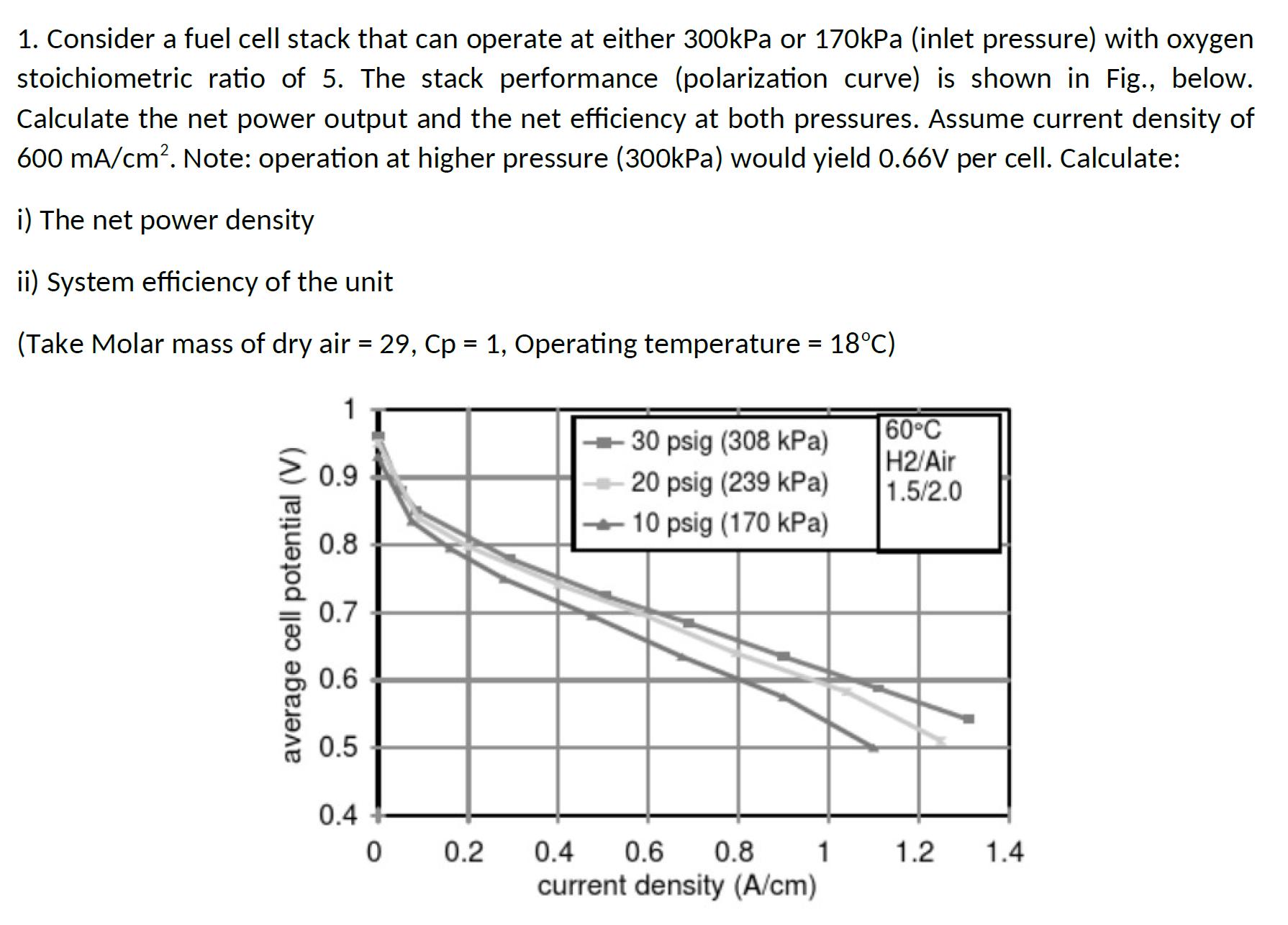
1. Consider a fuel cell stack that can operate at either 300kPa or 170kPa (inlet pressure) with oxygen stoichiometric ratio of 5. The stack performance (polarization curve) is shown in Fig., below. Calculate the net power output and the net efficiency at both pressures. Assume current density of 600 mA/cm. Note: operation at higher pressure (300kPa) would yield 0.66V per cell. Calculate: i) The net power density ii) System efficiency of the unit (Take Molar mass of dry air = 29, Cp = 1, Operating temperature = 18C) 60C 30 psig (308 kPa) average cell potential (V) 0.9 20 psig (239 kPa) H2/Air 1.5/2.0 10 psig (170 kPa) 0.8 0.7 0.6 0.5 0.4 0 0.2 0.6 0.4 current density (A/cm) 0.8 1 1.2 1.4
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
4th edition
978-1118431221, 9781119192138, 1118431227, 1119192137, 978-1119498759
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



