Question
The elementary liquid phase series reaction, ABC, is carried out in a PFR and CSTR where k = 0.5 min, k = 0.2 min,
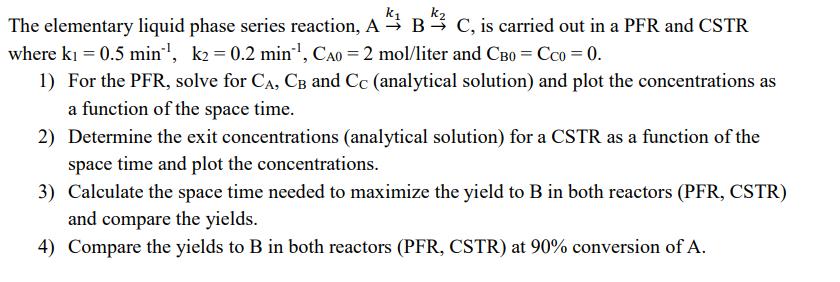

The elementary liquid phase series reaction, ABC, is carried out in a PFR and CSTR where k = 0.5 min, k = 0.2 min, CA0 = 2 mol/liter and CB0 = CC0 = 0. 1) For the PFR, solve for CA, CB and Cc (analytical solution) and plot the concentrations as a function of the space time. 2) Determine the exit concentrations (analytical solution) for a CSTR as a function of the space time and plot the concentrations. 3) Calculate the space time needed to maximize the yield to B in both reactors (PFR, CSTR) and compare the yields. 4) Compare the yields to B in both reactors (PFR, CSTR) at 90% conversion of A. 5) If you cannot solve the entire problem analytically (for example on an exam!), how would you estimate the selectivity in CSTR compared to PFR (for example at high conversion of 80%). 6) Plot CB as a function of XA for both reactors (PFR, CSTR).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



