Question
For the following elementary aqueous phase reactions (D is a desired product and U is undesired) k1 A D k2 A U K3 DU
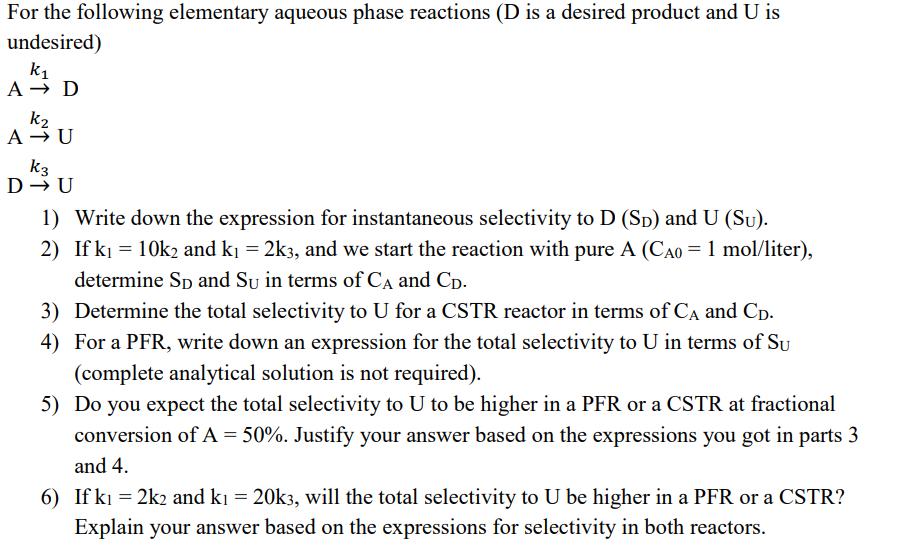
For the following elementary aqueous phase reactions (D is a desired product and U is undesired) k1 A D k2 A U K3 DU 1) Write down the expression for instantaneous selectivity to D (SD) and U (SU). 2) If k = 10k2 and k = 2k3, and we start the reaction with pure A (CA0 = 1 mol/liter), determine SD and Su in terms of CA and CD. 3) Determine the total selectivity to U for a CSTR reactor in terms of CA and CD. 4) For a PFR, write down an expression for the total selectivity to U in terms of Su (complete analytical solution is not required). 5) Do you expect the total selectivity to U to be higher in a PFR or a CSTR at fractional conversion of A = 50%. Justify your answer based on the expressions you got and 4. 6) If k = 2k2 and k = 20k3, will the total selectivity to U be higher in a PFR or a CSTR? Explain your answer based on the expressions for selectivity in both reactors.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



