Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Consider the hydrolysis of acetic anhydride carried out in aqueous solution in a PFR reactor: (CH3CO)2O+H2O2CH3COOH The t0=15C and AC0=0.03mol/L. Calculate the volume required
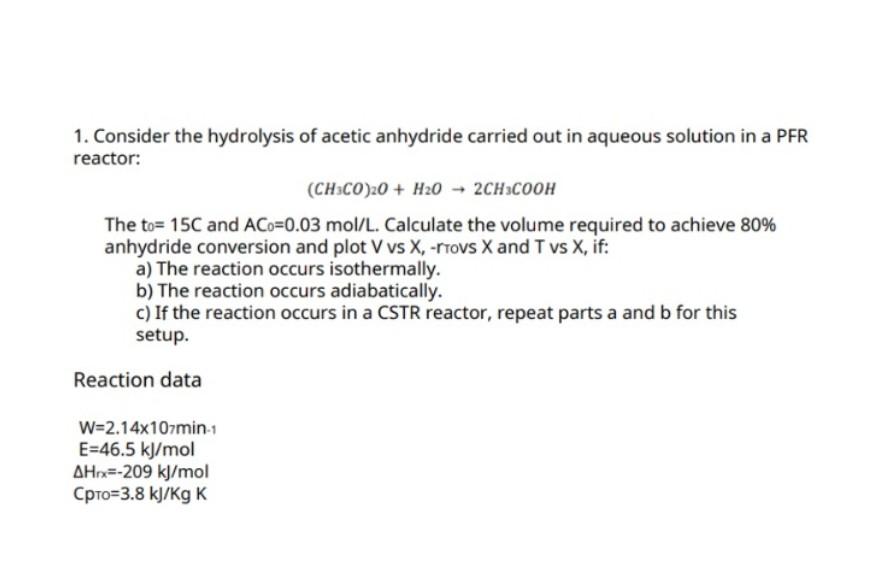
1. Consider the hydrolysis of acetic anhydride carried out in aqueous solution in a PFR reactor: (CH3CO)2O+H2O2CH3COOH The t0=15C and AC0=0.03mol/L. Calculate the volume required to achieve 80% anhydride conversion and plot V vs X, -rrovs X and T vs X, if: a) The reaction occurs isothermally. b) The reaction occurs adiabatically. c) If the reaction occurs in a CSTR reactor, repeat parts a and b for this setup. Reaction data W=2.14107min1E=46.5kJ/molH1x=209kJ/molC=3.8kJ/KgK
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


