Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1.- Consider the production of compound C according to the following kinetics, all reactions being exothermic. 2 3 D+E These reactions are carried out in
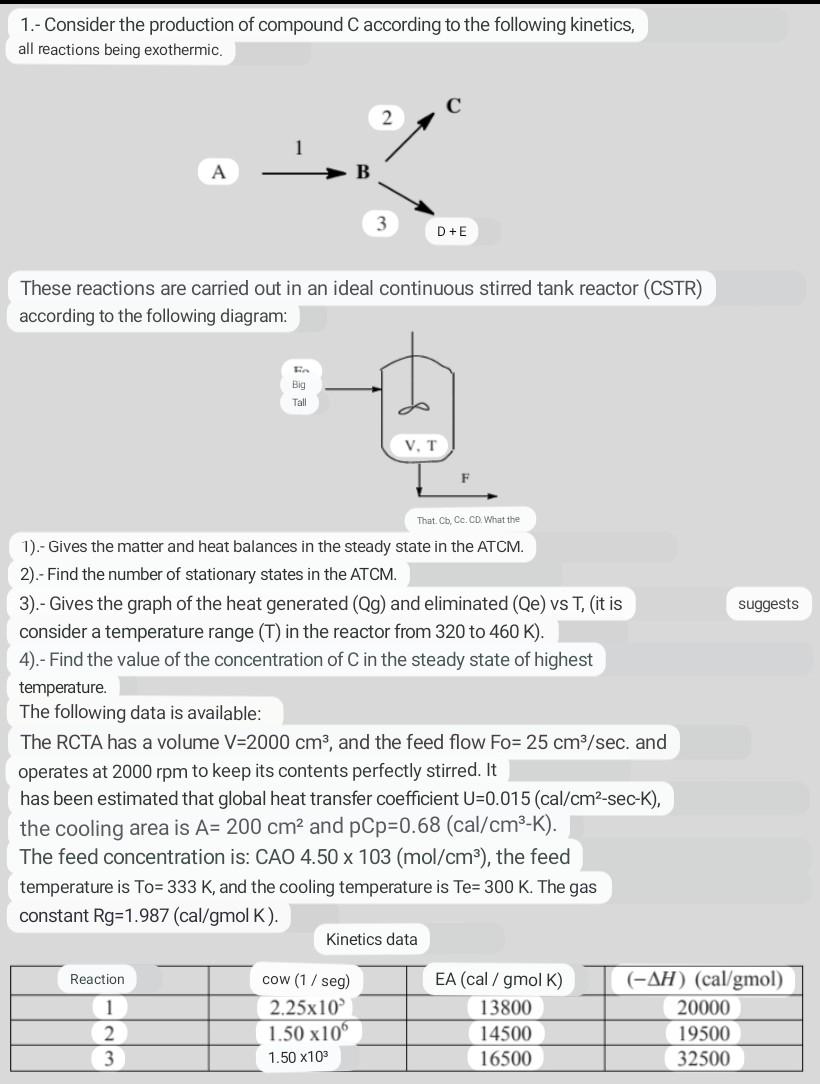
1.- Consider the production of compound C according to the following kinetics, all reactions being exothermic. 2 3 D+E These reactions are carried out in an ideal continuous stirred tank reactor (CSTR) according to the following diagram: Big Tall V. T F That Cb, Co. CD. What the suggests 1).- Gives the matter and heat balances in the steady state in the ATCM. 2).- Find the number of stationary states in the ATCM. 3).- Gives the graph of the heat generated (Qg) and eliminated (Qe) vs T, (it is consider a temperature range (T) in the reactor from 320 to 460 K). 4).- Find the value of the concentration of C in the steady state of highest temperature. The following data is available: The RCTA has a volume V=2000 cm3, and the feed flow Fo= 25 cm/sec. and operates at 2000 rpm to keep its contents perfectly stirred. It has been estimated that global heat transfer coefficient U=0.015 (cal/cm2-sec-K), the cooling area is A= 200 cm2 and pCp=0.68 (cal/cm3-K). The feed concentration is: CAO 4.50 x 103 (mol/cm), the feed temperature is To=333 K, and the cooling temperature is Te= 300 K. The gas constant Rg=1.987 (cal/gmol K). Kinetics data Reaction 1 2 3 cow (1/ seg) 2.25x10 1.50 x10 1.50 x103 EA (cal/gmol K) 13800 14500 16500 (-AH) (cal/gmol) 20000 19500 32500
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


