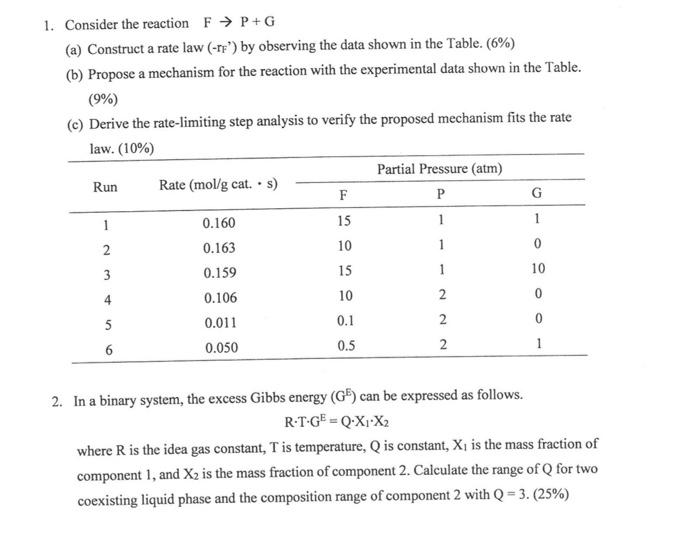
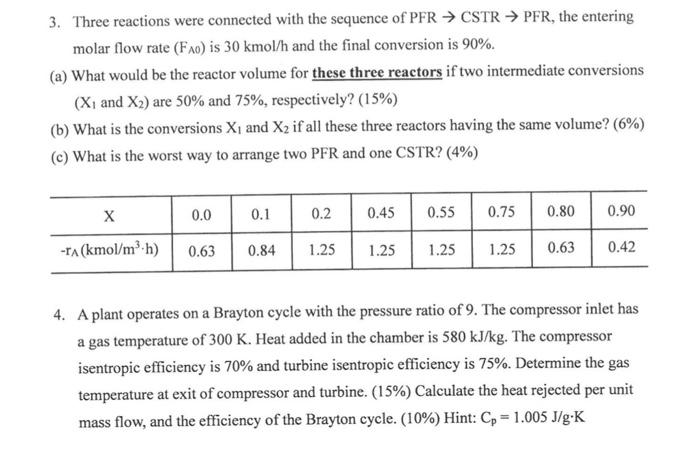
1. Consider the reaction FP+G (a) Construct a rate law (rF ') by observing the data shown in the Table. (6%) (b) Propose a mechanism for the reaction with the experimental data shown in the Table. (9%) (c) Derive the rate-limiting step analysis to verify the proposed mechanism fits the rate 1... 110% 2. In a binary system, the excess Gibbs energy (GE) can be expressed as follows. RTGE=QX1X2 where R is the idea gas constant, T is temperature, Q is constant, X1 is the mass fraction of component 1 , and X2 is the mass fraction of component 2. Calculate the range of Q for two coexisting liquid phase and the composition range of component 2 with Q=3.(25%) 3. Three reactions were connected with the sequence of PFR CSTR PFR, the entering molar flow rate (FA0) is 30kmol/h and the final conversion is 90%. (a) What would be the reactor volume for these three reactors if two intermediate conversions (X1 and X2) are 50% and 75%, respectively? (15%) (b) What is the conversions X1 and X2 if all these three reactors having the same volume? (6\%) (c) What is the worst way to arrange two PFR and one CSTR? (4\%) 4. A plant operates on a Brayton cycle with the pressure ratio of 9. The compressor inlet has a gas temperature of 300K. Heat added in the chamber is 580kJ/kg. The compressor isentropic efficiency is 70% and turbine isentropic efficiency is 75%. Determine the gas temperature at exit of compressor and turbine. (15%) Calculate the heat rejected per unit mass flow, and the efficiency of the Brayton cycle. (10\%) Hint: Cp=1.005J/gK 1. Consider the reaction FP+G (a) Construct a rate law (rF ') by observing the data shown in the Table. (6%) (b) Propose a mechanism for the reaction with the experimental data shown in the Table. (9%) (c) Derive the rate-limiting step analysis to verify the proposed mechanism fits the rate 1... 110% 2. In a binary system, the excess Gibbs energy (GE) can be expressed as follows. RTGE=QX1X2 where R is the idea gas constant, T is temperature, Q is constant, X1 is the mass fraction of component 1 , and X2 is the mass fraction of component 2. Calculate the range of Q for two coexisting liquid phase and the composition range of component 2 with Q=3.(25%) 3. Three reactions were connected with the sequence of PFR CSTR PFR, the entering molar flow rate (FA0) is 30kmol/h and the final conversion is 90%. (a) What would be the reactor volume for these three reactors if two intermediate conversions (X1 and X2) are 50% and 75%, respectively? (15%) (b) What is the conversions X1 and X2 if all these three reactors having the same volume? (6\%) (c) What is the worst way to arrange two PFR and one CSTR? (4\%) 4. A plant operates on a Brayton cycle with the pressure ratio of 9. The compressor inlet has a gas temperature of 300K. Heat added in the chamber is 580kJ/kg. The compressor isentropic efficiency is 70% and turbine isentropic efficiency is 75%. Determine the gas temperature at exit of compressor and turbine. (15%) Calculate the heat rejected per unit mass flow, and the efficiency of the Brayton cycle. (10\%) Hint: Cp=1.005J/gK