Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Consider the rovibrational spectrum for the v = 0 v = 1 vibrational transition of H35 Cl below, assuming the rotational constant is
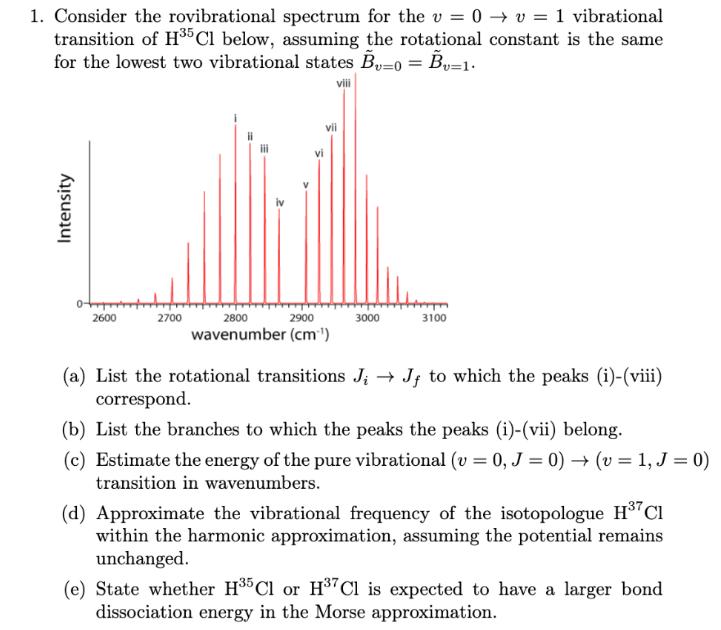
1. Consider the rovibrational spectrum for the v = 0 v = 1 vibrational transition of H35 Cl below, assuming the rotational constant is the same for the lowest two vibrational states B=0 = Bv=1. viii Intensity 0+ vii 2600 2700 2900 2800 wavenumber (cm) 3000 3100 (a) List the rotational transitions J; J; to which the peaks (i)-(viii) correspond. (b) List the branches to which the peaks the peaks (i)-(vii) belong. (c) Estimate the energy of the pure vibrational (v = 0, J = 0) (v = 1, J = 0) transition in wavenumbers. (d) Approximate the vibrational frequency of the isotopologue H37 CI within the harmonic approximation, assuming the potential remains unchanged. (e) State whether H35 Cl or H37 Cl is expected to have a larger bond dissociation energy in the Morse approximation.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


