Question
1. Determine the reaction order and specific reaction rate from the following data. t (min) 0 5 15 25 30 CA(mol/dm') 10 1.6 1.4
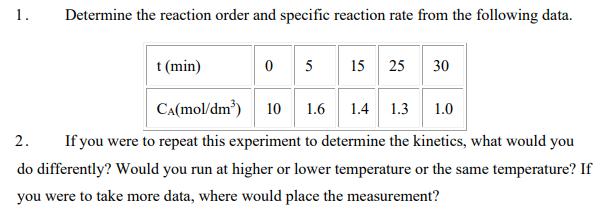
1. Determine the reaction order and specific reaction rate from the following data. t (min) 0 5 15 25 30 CA(mol/dm') 10 1.6 1.4 1.3 1.0 2. If you were to repeat this experiment to determine the kinetics, what would you do differently? Would you run at higher or lower temperature or the same temperature? If you were to take more data, where would place the measurement?
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Date Page Now easiest metnod is the kCAn raprical method dt enkhunCA dt a yamz on ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction To Statistical Investigations
Authors: Beth L.Chance, George W.Cobb, Allan J.Rossman Nathan Tintle, Todd Swanson Soma Roy
1st Edition
1118172140, 978-1118172148
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



