Question
1. Ethanol (1) and benzene (2) form azeotropic mixtures. a. From the limited data below, estimate A in the one-parameter Margules equation. Use all
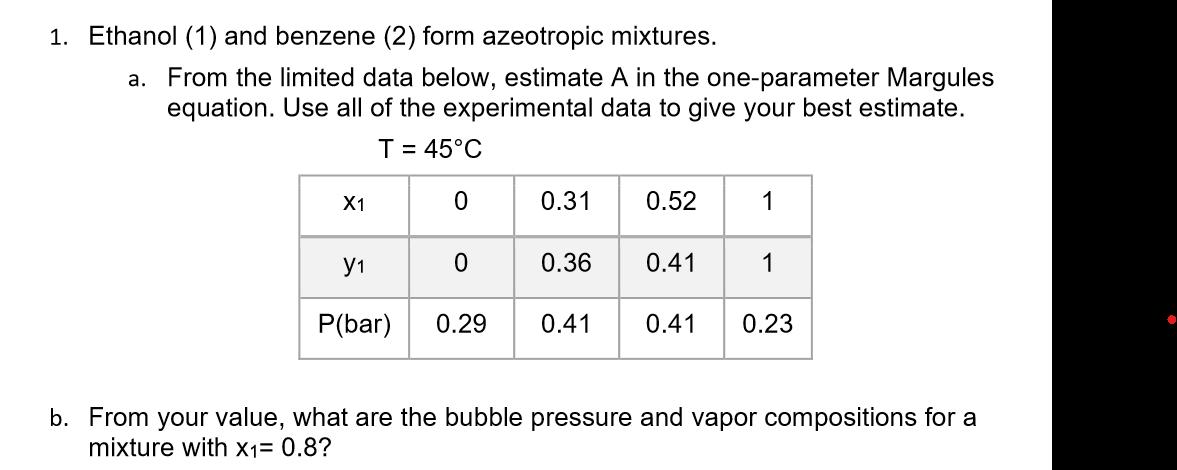
1. Ethanol (1) and benzene (2) form azeotropic mixtures. a. From the limited data below, estimate A in the one-parameter Margules equation. Use all of the experimental data to give your best estimate. T = 45C X1 1 0 0.31 0.52 1 0.36 0.41 1 P(bar) 0.29 0.41 0.41 0.23 b. From your value, what are the bubble pressure and vapor compositions for a mixture with x1= 0.8?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introductory Chemical Engineering Thermodynamics
Authors: J. Elliott, Carl Lira
2nd Edition
0136068545, 978-0136068549
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



