Ethanol(1) + benzene(2) form azeotropic mixtures. (a) From the limited data below at 45 C, it is
Question:
Ethanol(1) + benzene(2) form azeotropic mixtures.
(a) From the limited data below at 45 °C, it is desired to estimate the constant A for the one-term Margules equation, GE/RT = Ax1x2. Use all of the experimental data to give your best estimate.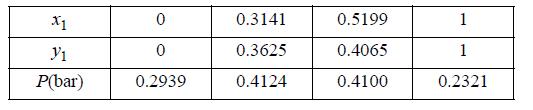
(b) From your value, what are the bubble pressure and vapor compositions for a mixture with x1 = 0.8?
Transcribed Image Text:
X1 Y1 P(bar) 0 0 0.2939 0.3141 0.3625 0.4124 0.5199 0.4065 0.4100 1 1 0.2321
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
To estimate the constant A for the oneterm Margules equation GERT Ax1x2 we can use the given experim...View the full answer

Answered By

Diane Joyce Pastorin
Please accept my enthusiastic application to solutioninn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group.
4.60+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
It is desired to estimate the average consumer spending on durable goods, annually, in a defined geographical region. Spending patterns are influenced by disposable income. Based on county tax...
-
It is desired to enrich the partial pressure of hydrogen in a hydrogen-nitrogen gas mixture for which the partial pressures of both gases are 0.1013 MPa (1 atm). It has been proposed to accomplish...
-
The cooled exhaust gas from a reactor that makes silicon by the chemical vapor deposition of trichlorosilane contains 8.0 mole% anhydrous HC1 vapor and 92.0 mole % hydrogen (H 2 ) gas at 25°C....
-
Problem 4.2 Ask the user to enter his/her age, with the prompt: "How old are you? Please enter your age as a number between 0 and 120. -> ". Check that what was entered is a number between 0 and 120....
-
Give two examples other than oligopoly that show how the prisoners dilemma helps to explain behavior.
-
YourFire, Inc., is a small business owned by Curt and Julie Robards. Based in Brisbane, Australia, YourFire manufactures and sells a lightweight camping stove called the YourFire. Curt, who...
-
Describe one way that marketers can use posttesting to measure the effectiveness of an advertising campaign. For which kind of product is this method most useful? Why is it challenging to track the...
-
Waymon Co. has net sales of $100,000, cost of goods sold of $70,000, and operating expenses of $18,000. What is its gross profit?
-
(True or False): A private family-owned business has an older family member who wants to retire and sell her shares. The problem is the family member owns a significant part of the family business...
-
Fit the data from problem 11.11 to the following model by regression over all points, and compare with the experimental data on the same plot, using: (a) One-parameter Margules equation (b)...
-
A liquid mixture of 50 mol% chloroform(1) and 50% 1,4-dioxane(2) at 0.1013 MPa is metered into a flash drum through a valve. The mixture flashes into two phases inside the drum where the pressure and...
-
A limitation on how many spectra per second can be recorded by a time-of-flight mass spectrometer is the time it takes the slowest ions to go from the source to the detector. Suppose we want to scan...
-
Brice Looney owns a small retail ice cream parlor. He is considering expanding the business and has identified two attractive alternatives. One involves purchasing a machine that would enable Mr....
-
A positively charged particle initially at rest on the ground moves \(4.0 \mathrm{~m}\) upward in \(2.00 \mathrm{~s}\). If the particle has a chargeto-mass ratio of \(10 \mu \mathrm{C} / \mathrm{g}\)...
-
Central States Telecom provides communication services in Iowa, Nebraska, the Dakotas, and Montana. Central States purchased goodwill as part of the acquisition of Sheldon Wireless Company, which had...
-
Shown below is selected information from the financial records of Merris Corporation as of December 31: Required a. Determine which of the above items will appear on the statement of cash flows and...
-
Pippa runs a photographic studio specializing in black and white portrait photography. Clients book a one hour studio session and are entitled to receive two large photographs of their choice from...
-
The following information relates to Zulu Company's accounts receivable for 2017: Accounts receivable, 1/1/2017.........................$ 750,000 Credit sales for...
-
Is that Yelp review real or fake? The article A Framework for Fake Review Detection in Online Consumer Electronics Retailers (Information Processing and Management 2019: 12341244) tested five...
-
An ideal gas with constant heat capacities enters a converging/diverging nozzle with negligible velocity. If it expands isentropically within the nozzle, show that the throat velocity is given by:...
-
As suggested by Fig. 3.1, the slope of the sublimation curve at the triple point is generally greater than that of the vaporization curve at the same state. Rationalize this observation. Note that...
-
As suggested by Fig. 3.1, the slope of the sublimation curve at the triple point is generally greater than that of the vaporization curve at the same state. Rationalize this observation. Note that...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...

Study smarter with the SolutionInn App


