An ideal gas with constant heat capacities enters a converging/diverging nozzle with negligible velocity. If it expands
Question:
An ideal gas with constant heat capacities enters a converging/diverging nozzle with negligible velocity. If it expands isentropically within the nozzle, show that the throat velocity is given by: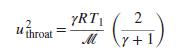 where T1 is the temperature of the gas entering the nozzle, ℳ is the molar mass, and R is the molar gas constant.
where T1 is the temperature of the gas entering the nozzle, ℳ is the molar mass, and R is the molar gas constant.
Transcribed Image Text:
yRT| throat r+1, (4)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
The velocity of a convergingdiverging nozzle can be found using the isentropic relation which states ...View the full answer

Answered By

Dulal Roy
As a tutor, I have gained extensive hands-on experience working with students one-on-one and in small group settings. I have developed the ability to effectively assess my students' strengths and weaknesses, and to customize my teaching approach to meet their individual needs.
I am proficient at breaking down complex concepts into simpler, more digestible pieces, and at using a variety of teaching methods (such as visual aids, examples, and interactive exercises) to engage my students and help them understand and retain the material.
I have also gained a lot of experience in providing feedback and guidance to my students, helping them to develop their problem-solving skills and to become more independent learners. Overall, my hands-on experience as a tutor has given me a deep understanding of how to effectively support and encourage students in their learning journey.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
An ideal gas with constant heat capacities undergoes a change of state from conditions T1, P1 to conditions T2, P2. Determine (H (J mol-1) and (S (J mol-1 K-1) for one of the following cases. (a) T1...
-
A mapping T: R2 R2 is given by Where Show that T rotates every vector v ε R2 counterclockwise about the origin through angle 0. Express v using polar coordinates, And use the identities...
-
An ideal gas with the adiabatic exponent goes through a cycle (Fig. 2.3) within which the absolute temperature varies -fold. Find the efficiency of this cycle.
-
Write a program to Sort the list U,N,I,V,E,R,S,I,T,Y in alphabetical order by Bubble sort I want the complete solution steps with the solution to the question
-
Name four of the positive participation skills required of team members.
-
Analyze Take- Twos 1998-2000 financial data included in Exhibit 1. Compute the following financial ratios for each of those years: age of accounts receivable, age of inventory, gross profit...
-
Briefly describe when you would use regression analysis and correlation analysis, using examples to illustrate your answer. LO9
-
Overnight Laundry is considering the purchase of a new pressing machine that would cost $100,000 and would produce incremental cash flows of $25,000 annually for 6 years. The machine has a terminal...
-
\ table [ [ Currency , \ table [ [ Expected ] , [ Exchange rate ] ] , \ table [ [ Range of possible ] , [ exchange rates ] ] ] , [ US dollar, 3 . 7 5 SAR, 3 . 7 5 SAR to 3 . 7 5 SAR ] , [ Japanese...
-
In Malaysia, certain job sectors, like IT and customer services, are increasingly dependent on specialist human resource providers as a common source of trained manpower. Companies like Manpower...
-
A gas enters a converging nozzle at pressure P 1 with negligible velocity, expands isentropically in the nozzle, and discharges into a chamber at pressure P 2 . Sketch graphs showing the velocity at...
-
As suggested by Fig. 3.1, the slope of the sublimation curve at the triple point is generally greater than that of the vaporization curve at the same state. Rationalize this observation. Note that...
-
A poultry scientist was studying various dietary additives to increase the rate at which chickens gain weight. One of the potential additives was studied by creating a new diet that consisted of a...
-
This case study is based on a fictional character on NBC's The Office. Michael is the central character of the series, serving as the Regional Manager of the Scranton branch of a paper distribution...
-
What is the significance of a balance sheet in understanding a firm's financial position? How do changes on the right side of the balance sheet (liabilities and equity) impact a company's financial...
-
A current event analysis where the article must focus on a management concepts). You will read the article and then provide an analysis of the subject matter discussed. The article should complement...
-
Given an exponential distribution with =20, what is the probability that the arrival time is a. less than X=0.2? b. greater than X = 0.2? c. between X=0.2 and X 0.3? d. less than X=0.2 or greater...
-
Choose at least two measures of employee attitudes. Discuss them and tell me about your discussion. Which group you believe are the most effective and efficient measures? Why? 2) Discuss turnover,...
-
Wolz Company, a small business, has had a defined benefit pension plan for its employees for several years. At the beginning of 2019, Wolz amended the pension plan; this amendment provides for...
-
Suppose that you are part of a virtual team and must persuade other team members on an important matter (such as switching suppliers or altering the project deadline). Assuming that you cannot visit...
-
Liquid/vapor saturation pressure P sat is often represented as a function of temperature by the Antoine equation, which can be written in the form: Here, parameters a, b, and c are substance-specific...
-
Electric current is the fundamental SI electrical dimension, with the ampere (A) as its unit. Determine units for the following quantities as combinations of fundamental SI units. (a) Electric power...
-
An incompressible fluid ( = constant) is contained in an insulated cylinder fitted with a frictionless piston. Can energy as work be transferred to the fluid? What is the change in internal energy of...
-
Crane, Inc., a resort management company, is refurbishing one of its hotels at a cost of $6,794,207. Management expects that this will lead to additional cash flows of $1,560,000 for the next six...
-
Match each of the following transactions with the applicable internal control principle that is being violated
-
Vaughn Company sells two types of pumps. One is large and is for commercial use. The other is smaller and is used in residential swimming pools. The following inventory data is available for the...

Study smarter with the SolutionInn App


