Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. For a certain binary system, the liquid phase obeys the van Laar activity coefficient model. For a vapour-liquid situation at a certain temperature, the
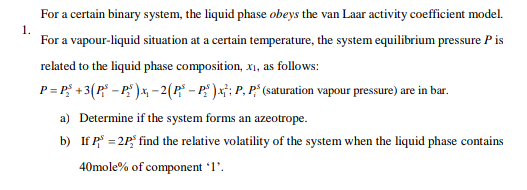
1. For a certain binary system, the liquid phase obeys the van Laar activity coefficient model. For a vapour-liquid situation at a certain temperature, the system equilibrium pressure Pis related to the liquid phase composition, X1, as follows: P=P* +3(P* - P.) , - 2(P - P:" ) &?; P, P* (saturation vapour pressure) are in bar. a) Determine if the system forms an azeotrope. b) If p" = 2p% find the relative volatility of the system when the liquid phase contains 40mole% of component '1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


