Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) For a hypothetical A - B regular solution at 1250K, interatomic force parameters is given as =1500. For XB=0.20.40.60.8 compositions: a) Calculate the enthalpy
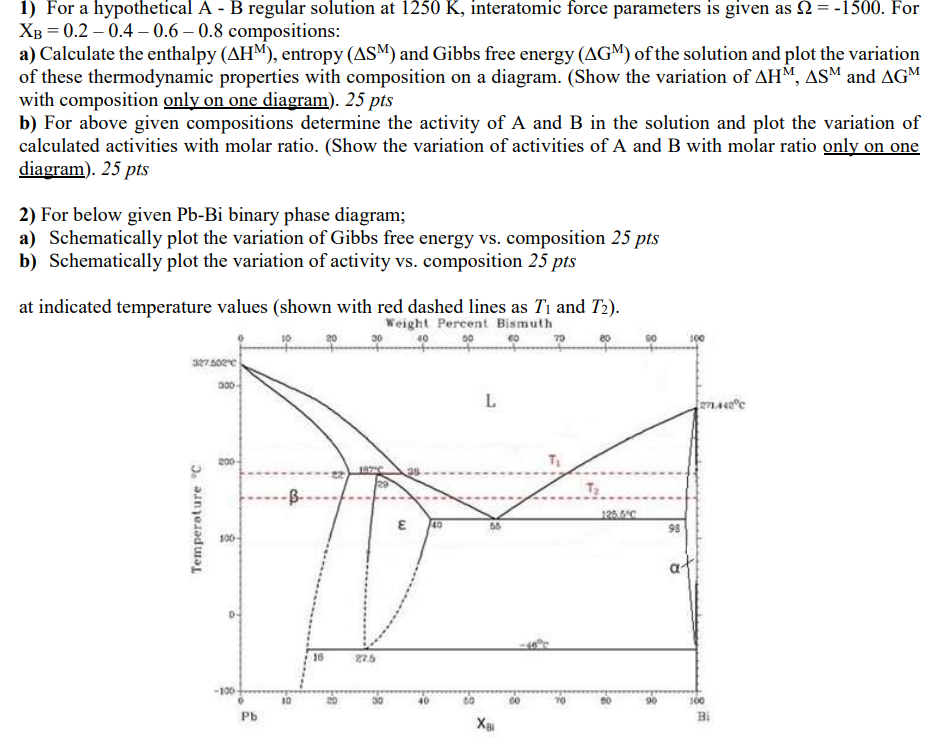 1) For a hypothetical A - B regular solution at 1250K, interatomic force parameters is given as =1500. For XB=0.20.40.60.8 compositions: a) Calculate the enthalpy (HM), entropy (SM) and Gibbs free energy (GM) of the solution and plot the variation of these thermodynamic properties with composition on a diagram. (Show the variation of HM,SM and GM with composition only on one diagram ).25 pts b) For above given compositions determine the activity of A and B in the solution and plot the variation of calculated activities with molar ratio. (Show the variation of activities of A and B with molar ratio only on one diagram ).25pts 2) For below given PbBi binary phase diagram; a) Schematically plot the variation of Gibbs free energy vs. composition 25pts b) Schematically plot the variation of activity vs. composition 25pts at indicated temperature values (shown with red dashed lines as T1 and T2 )
1) For a hypothetical A - B regular solution at 1250K, interatomic force parameters is given as =1500. For XB=0.20.40.60.8 compositions: a) Calculate the enthalpy (HM), entropy (SM) and Gibbs free energy (GM) of the solution and plot the variation of these thermodynamic properties with composition on a diagram. (Show the variation of HM,SM and GM with composition only on one diagram ).25 pts b) For above given compositions determine the activity of A and B in the solution and plot the variation of calculated activities with molar ratio. (Show the variation of activities of A and B with molar ratio only on one diagram ).25pts 2) For below given PbBi binary phase diagram; a) Schematically plot the variation of Gibbs free energy vs. composition 25pts b) Schematically plot the variation of activity vs. composition 25pts at indicated temperature values (shown with red dashed lines as T1 and T2 ) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


