Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a titration provides a chemist with information regarding the equivalence point of a reac- tion. How is this information useful? Here are two examples.
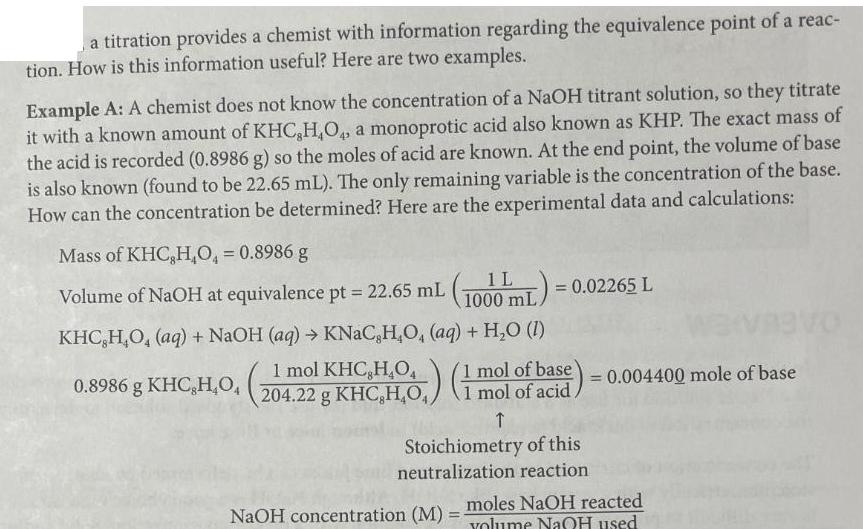

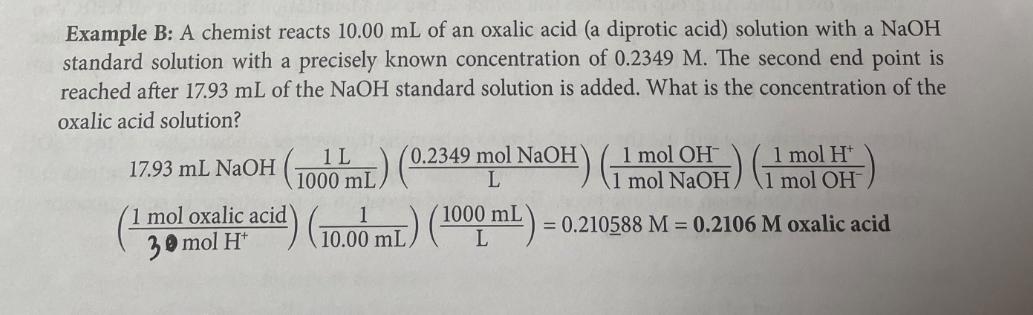
a titration provides a chemist with information regarding the equivalence point of a reac- tion. How is this information useful? Here are two examples. Example A: A chemist does not know the concentration of a NaOH titrant solution, so they titrate it with a known amount of KHC,H,O, a monoprotic acid also known as KHP. The exact mass of the acid is recorded (0.8986 g) so the moles of acid are known. At the end point, the volume of base is also known (found to be 22.65 mL). The only remaining variable is the concentration of the base. How can the concentration be determined? Here are the experimental data and calculations: Mass of KHC,H,O = 0.8986 g = 0.02265 L Volume of NaOH at equivalence pt = 22.65 mL (1000 mL) = 0 KHC,H,O, (aq) + NaOH (aq) KNaC,H,O, (aq) + H2O (I) HO (1 mol of base) = 0.004400 mole of base 0.8986 g KHC,H,O, (21 mol KCC10) (1 mol of acid Stoichiometry of this neutralization reaction NaOH concentration (M) = moles NaOH reacted volume NaQH used 0.00440016 moles NaOH = 0.194267 M = 0.1943 M NaOH Spot Check LLT.4 0.02265 L Visit your digital lab manual for Interactive Spot Checks! What is the concentration of an NaOH solution if it reacts with 0.7439 g of KHC,H,O, and the equivalence point is reached after 19.23 mL of base has been added? Example B: A chemist reacts 10.00 mL of an oxalic acid (a diprotic acid) solution with a NaOH standard solution with a precisely known concentration of 0.2349 M. The second end point is reached after 17.93 mL of the NaOH standard solution is added. What is the concentration of the oxalic acid solution? mol 17.93 mL NaOH (1000 mL) (0.2349 mol NaOH) (1 mol OHH) (11) (1 mol oxalic acid) (1000 mL) (1000 mL) 30 mol H = 0.210588 M = 0.2106 M oxalic acid
Step by Step Solution
★★★★★
3.41 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


