Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. for standardization of kMnO4 reduction half reaction oxidation half reaction overall reaction 2. Based on the stoichiometry of the overall reaction, show how the
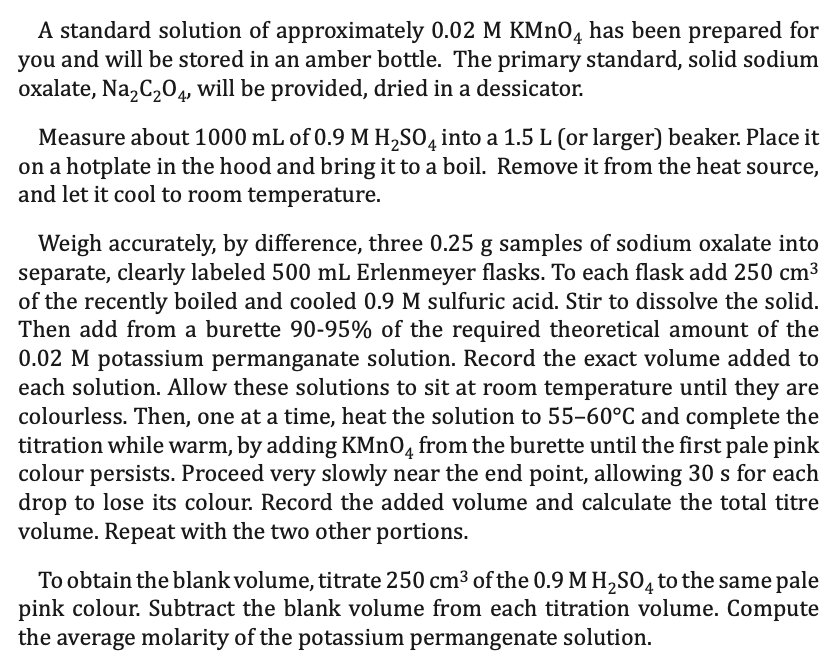
1. for standardization of kMnO4
reduction half reaction
oxidation half reaction
overall reaction
2. Based on the stoichiometry of the overall reaction, show how the mass of your primary standard is related to the concentration of permanganate.
3. What is the purpose of the blank titration?
4. Why was 9095% of the required permanganate added in one batch to the oxalate solution and then left standing for 15 min?
A standard solution of approximately 0.02 M KMnO4 has been prepared for you and will be stored in an amber bottle. The primary standard, solid sodium oxalate, Na2C204, will be provided, dried in a dessicator. Measure about 1000 mL of 0.9 M H2SO4 into a 1.5 L (or larger) beaker. Place it on a hotplate in the hood and bring it to a boil. Remove it from the heat source, and let it cool to room temperature. Weigh accurately, by difference, three 0.25 g samples of sodium oxalate into separate, clearly labeled 500 mL Erlenmeyer flasks. To each flask add 250 cm3 of the recently boiled and cooled 0.9 M sulfuric acid. Stir to dissolve the solid. Then add from a burette 90-95% of the required theoretical amount of the 0.02 M potassium permanganate solution. Record the exact volume added to each solution. Allow these solutions to sit at room temperature until they are colourless. Then, one at a time, heat the solution to 55-60C and complete the titration while warm, by adding KMnO4 from the burette until the first pale pink colour persists. Proceed very slowly near the end point, allowing 30 s for each drop to lose its colour. Record the added volume and calculate the total titre volume. Repeat with the two other portions. To obtain the blank volume, titrate 250 cm3 of the 0.9 M H2SO4 to the same pale pink colour. Subtract the blank volume from each titration volume. Compute the average molarity of the potassium permangenate solutionStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


