Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. For the irreversible gas-phase dissociation of the dimer A, A 2A Determine the CSTR volume necessary to achieve 80% conversion and produce 1000 g
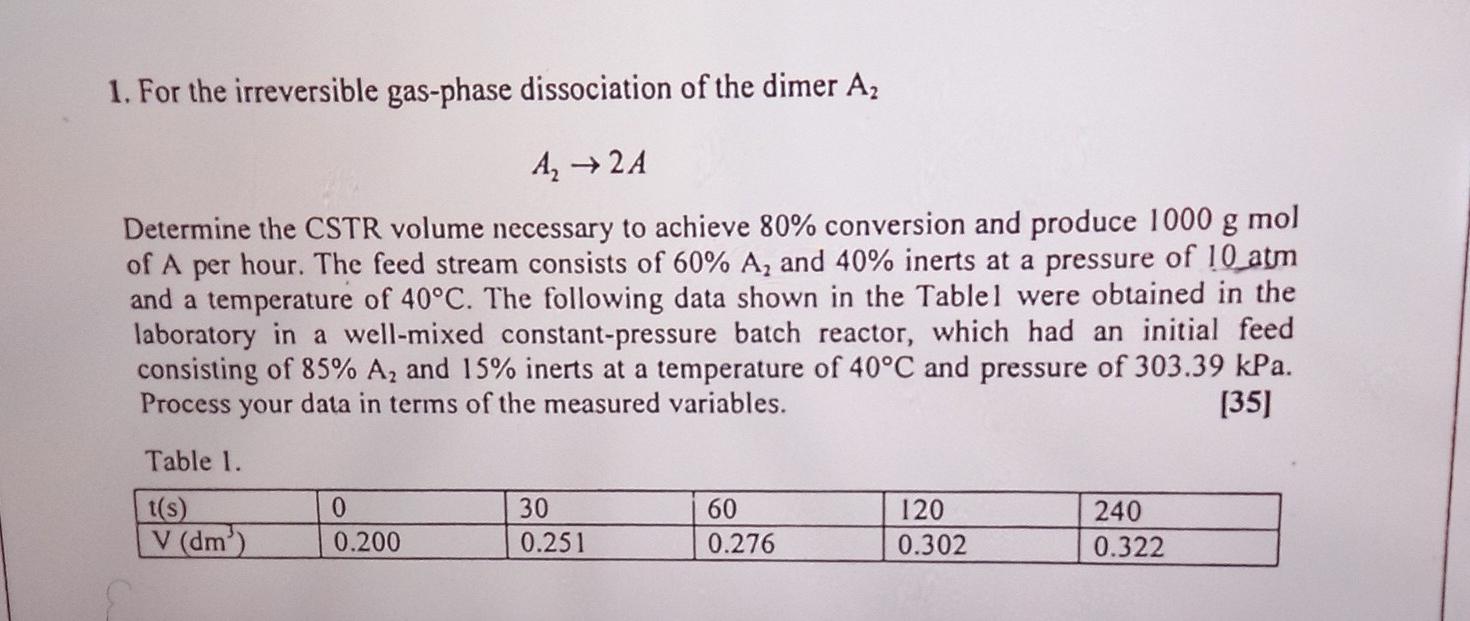
1. For the irreversible gas-phase dissociation of the dimer A, A 2A Determine the CSTR volume necessary to achieve 80% conversion and produce 1000 g mol of A per hour. The feed stream consists of 60% A, and 40% inerts at a pressure of 10 atm and a temperature of 40C. The following data shown in the Tablel were obtained in the laboratory in a well-mixed constant-pressure batch reactor, which had an initial feed consisting of 85% A2 and 15% inerts at a temperature of 40C and pressure of 303.39 kPa. Process your data in terms of the measured variables. [35] Table 1. t(s) 30 60 120 240 V (dm) 0.200 0.251 0.276 0.302 0.322 0
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


