Question
1. How are elements arranged in the modern periodic table? 2. Name the three broad classes of elements? 3. Identify each element as a metal,
1. How are elements arranged in the modern periodic table?
2. Name the three broad classes of elements?
3. Identify each element as a metal, a metalloid, and non-metal.
Gold (Au) B. sulfur (S) C. silicon (Si) D. Barium (Ba)
4. Based on the following electron configurations. Identify each element as a representative element, transition metal, or noble gas. Identify the valence of each one of them?
A. 1s22s22p63s23p63d104s24p6
B. 1s22s22p63s23p63d64s2
C. 1s22s22p63s23p2
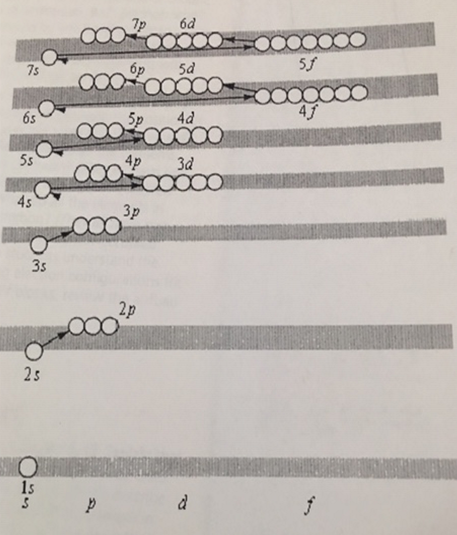
5. Using the figure below, write the electron configuration of the following
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


