Question
1. How many inner, outer, and valence electrons are present in an atom of each of the following elements? (a) O (b) Sn (c)
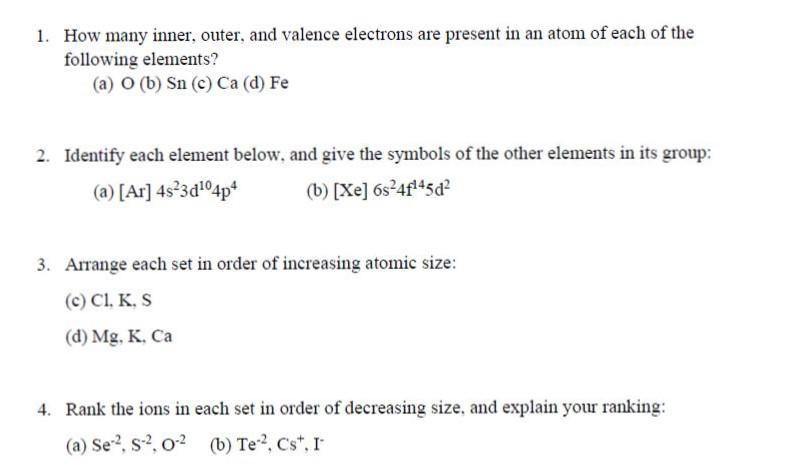
1. How many inner, outer, and valence electrons are present in an atom of each of the following elements? (a) O (b) Sn (c) Ca (d) Fe 2. Identify each element below, and give the symbols of the other elements in its group: (a) [Ar] 4s3d04p* (b) [Xe] 6s4f45d? 3. Arrange each set in order of increasing atomic size: (c) Cl, K, S (d) Mg, K. Ca 4. Rank the ions in each set in order of decreasing size, and explain your ranking: (a) Se2, s, 02 (b) Te2, Cs", I
Step by Step Solution
3.39 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
3 A S Cl K B Mg Ca K 4 A Se S O Because we know as we move down the group size inc...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



