Question
1. Hydrogen peroxide reacts with thiosulfate ion in slightlyacidic solution as follows: H 2 O 2 + 2S 2 O 3 2? + 2 H
1. Hydrogen peroxide reacts with thiosulfate ion in slightlyacidic solution as follows:
H2O2 + 2S2O32?+ 2 H+? 2H2O + S4O62?
The reaction rate is independent of the hydrogen ionconcentration in the pH range 4 to 6. The following data wereacquired at 25 ?C and pH 5.0. Initial concentrations:[H2O2] = 0.036 mol/L,[S2O32-] = 0.0204 mol/L
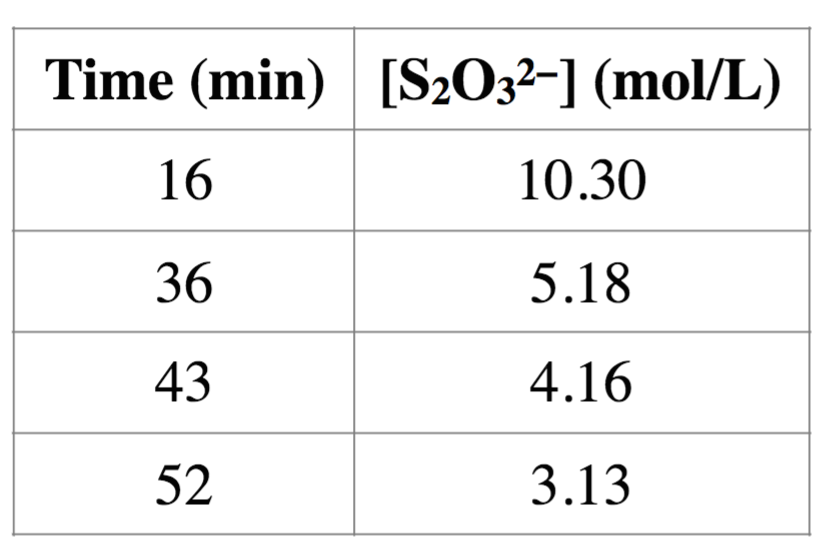
(a) What is the overall order of the reaction?
(b) What is the rate constant?
No information was given for [H2O2] for the course ofthe reaction
2. For the reaction, where M is an inert gas atom (such as He orAr),
O + NO + M ? NO2 + M
The rate constant exhibits the following temperaturebehavior:
at 300 K, k = 6 ? 109L2mol?2s?1; at 1000 K, k = 3 x1010 L2mol?2s?1.
Determine the activation energy and the prexponential factorusing the Arrhenius equation.
Time (min) [SO3-] (mol/L) 16 36 43 52 10.30 5.18 4.16 3.13
Step by Step Solution
3.57 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


