An acidified solution of hydrogen peroxide reacts with iodide ions. H 2 O 2 (aq) + 2H
Question:
An acidified solution of hydrogen peroxide reacts with iodide ions.
H2O2(aq) + 2H+(aq) + 2I–(aq) → 2H2O(l) + I2(aq)
The rate equation for this reaction is rate = [H2O2] [I–] The mechanism below has been proposed for this reaction.
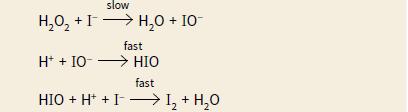
Explain why this mechanism is consistent with the rate equation.
slow H,0, +I> H,0 + IO H,0 + 10- fast H* + IO- > HIO fast HIO + H* + I -I, + H,0
Step by Step Answer:
The rate equation rate H2O2 I suggests that the reaction rate depends on both hydrogen peroxide and ...View the full answer



Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Related Video
Hydrogen peroxide can be used as a mild antiseptic to curb superficial skin infections such as athlete’s foot, but only in diluted quantities. To combat stinky feet, try soaking your feet in a solution of 1 part 3% hydrogen peroxide and 3 parts warm water for 15-20 minutes, then drying them thoroughly. This will kill odor-causing bacteria and soften your feet. To treat athlete\'s foot, you can use a similar solution, but only in diluted quantities, and soak your feet for 30 minutes. Hydrogen peroxide can also be used to keep vegetables fresh by adding 1/4 cup to a bowl of cold water, soaking the vegetables for 20-30 minutes, then draining, drying, and refrigerating them. Alternatively, you can spray vegetables with a solution of 3% hydrogen peroxide and let them stand for a few minutes before rinsing and drying. To keep leftover salad fresh, spray it with a solution of 1/2 cup water and 1 Tbsp. 3% hydrogen peroxide, drain, cover, and refrigerate.
Students also viewed these Sciences questions
-
The rate equation for the reaction between iodine and propanone is: rate = k[CH 3 COCH 3 ] [H + ] [I 2 ] 0 a. State the order of reaction with respect to iodine. b. State the overall order of...
-
Aldolase catalyzes the reaction ÎG°² for this reaction is 22.8 kJmol-1. In the cell at 37°C, the ÎG for this reaction is -5.9 kJmol-1. What is the ratio [GAP][DHAP]/[FBP]?...
-
When you calculated A in the rate equation for the reaction of kMnO 4 solution and H 2 C 2 O 4 solution. you assumed k had the same value under the conditions of determinations 1, 2, and 3. (a) What...
-
The CFO of the Jordan Microscope Corporation intentionally misclassified a downstream transportation expense in the amount of $575,000 as a product cost in an accounting period when the company made...
-
Consider population data with = 20 and = 2. (a) Compute the coefficient of variation. (b) Compute an 88.9% Chebyshev interval around the population mean.
-
Jays Flying Advertising, LLC, contracted with Big Bobs Burger restaurant to fly an advertisement above the Connecticut beaches. The advertisement offered $5,000 to any person who could swim from the...
-
Consider a model with date0 endowmentsyh0 and date0 consumption ch0. Suppose all investors have log utility, a common discount factor , and no date1 labor income. Show that, in a competitive...
-
Grand Slam Manufacturing Co. operates on a modified wage plan. During one week's operation, the following direct labor costs were incurred: The employees are machine operators. Piece rates vary with...
-
On May 27, Kick Off Inc. reacquired 75,000 shares of its common stock at $8 per share. On August 3, Kick off sold 50,000 of the reacquired shares at $11 per share. On November 14, Kick off sold the...
-
1. Write a brief memo to Zoe explaining the importance of data validation during the input process. 2. Suggest at least three specific data validation checks that might help reduce input errors. 3....
-
a. State which pairs of substances i to iv below might catalyse the reaction: S 2 O 8 2 (aq) + 2I (aq) 2SO 4 2 (aq) + I 2 (aq) Explain your answer. i. Ni 2+ (aq) / Ni(s) E = 0.25 V ii. Mn 3+ (aq)...
-
a. Write the rate equation for the acid-catalysed reaction of iodine with propanone. b. Use your rate equation and the information in Table 22.9 (experiment 1) to calculate a value for the rate...
-
Let X have density fx(x) = 25xe-5x for 0 < x and fx(x) = 0 otherwise. Find Var(X).
-
there are some solbeed with direct materials. this one says direct labor. any help would be appreciated, ive been stuck Chapter 9 Homework Save 1.5 6 H 305 Parker Plastic, Incorporated, manufactures...
-
Give examples of applications where pumps might be connected in series. Give examples of applications where pumps might be connected in parallel. Drawing on the conclusions of earlier exercises,...
-
a truck company has 2 trucks, which are hired out day by day. The average number of trucks hired on a day follows a distribution with mean 1 . 5 . Identify the distribution and then find the...
-
Designand drive selectionfor a hydrostaticapplication.Choose anypropelledequipmentwithopen or closedloop HST. Includethepayloadand/or anymachinefunctionrequirementsfor the mobileequipment.A sketch...
-
A two stage air compressor with ideal intercooler pressure and perfect intercooling (what does this mean?) compresses air from 1 bar to 16 bar at the rate of 5 m3/min. Mechanical efficiency of the...
-
Main Street Charities (MSC) is a not-for-profit organization that works in a large city to alleviate poverty. One of its programs is supported solely by private donations. In preparing the budget for...
-
In a paragraph of approximately 150-200 words, analyze a film or TV/Streaming Show poster of your choosing by focusing on the ways in which representations in the poster are gendered. Include an...
-
For each of the compounds below, locate the pattern we just learned (lone pair next to a Ï bond) and draw the appropriate resonance structure: a. b. c. d. e. f. g. h. NH2
-
Draw the resonance structure(s) for each of the compounds below: a. b. c. d.
-
The formalism of the Youngs modulus is sometimes used to calculate the reversible work involved in extending or compressing an elastic material. Assume a force F is applied to an elastic rod of...
-
Questien It Calraluta bae neark yoe cen atforal to berren
-
In calculating the net present value of a proposed project, the cash flows of the project should include a.) amortization of goodwill b.) interest expenses paid to bondholders c.) extra working...
-
If Yolanda's insurance company cancels her fire insurance policy after 204 days, how much of the $682.00 annual premium will she receive as a refund (in $)? (Round you answer to the nearest cent.) $

Study smarter with the SolutionInn App


