a. Write the rate equation for the acid-catalysed reaction of iodine with propanone. b. Use your rate
Question:
a. Write the rate equation for the acid-catalysed reaction of iodine with propanone.
b. Use your rate equation and the information in Table 22.9 (experiment 1) to calculate a value for the rate constant for this reaction.
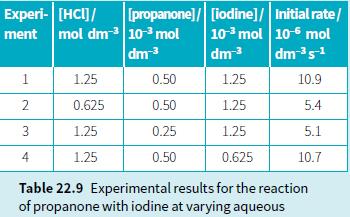
Transcribed Image Text:
Experi- [HCI]/ ment mol dm 10- mol dm 3 [propanone]/ [iodine]/ Initial rate/ 103 mol 10-6 mol dm3 dms1 1 1.25 0.50 1.25 10.9 2 0.625 0.50 1.25 5.4 1.25 0.25 1.25 5.1 4 1.25 0.50 0.625 10.7 Table 22.9 Experimental results for the reaction of propanone with iodine at varying aqueous 3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
A The reaction is T he reaction is first ...View the full answer

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
The rate equation for the reaction between iodine and propanone is: rate = k[CH 3 COCH 3 ] [H + ] [I 2 ] 0 a. State the order of reaction with respect to iodine. b. State the overall order of...
-
When you calculated A in the rate equation for the reaction of kMnO 4 solution and H 2 C 2 O 4 solution. you assumed k had the same value under the conditions of determinations 1, 2, and 3. (a) What...
-
Write the balanced equation for the reaction of sulfurous acid with dichromate ion.
-
Jacky Ma Ltd sells a single product called Alibaba. During 2020, 10,000 units were produced and 9,500 units were sold. There was no work-in-process inventory on 31 December 2020, that is the...
-
(a) Compute the coefficient of variation. (b) Compute a 75% Chebyshev interval around the sample mean.
-
Evelyn Kowalchuk, an eighty-eight-year-old widow, and her son, Peter, put their savings into accounts managed by Matthew Stroup. Later, they initiated an arbitration proceeding before the National...
-
Suppose the payoff of the market portfolio w m has k possible values. Denote these possible values by a1 < < ak. For convenience, suppose ai ai1 is the same number for each i. Suppose there is a...
-
Find the position of the center of mass of the system of the sun and Jupiter. (Since Jupiter is more massive than the rest of the planets combined, this is essentially the position of the center of...
-
2. July, 2018, trial balance shows Unearned Rent Revenue $5,100 in credit side. Two- thirds of the Unearned Rent Revenue has not been earned during the month. What will be the adjusting entry for the...
-
The Terminus Hotel, a 200-room facility located in a medieval city in Southern Spain. As consequence of poor management and old-fashioned interior design. the hotel experienced slumping demand since...
-
An acidified solution of hydrogen peroxide reacts with iodide ions. H 2 O 2 (aq) + 2H + (aq) + 2I (aq) 2H 2 O(l) + I 2 (aq) The rate equation for this reaction is rate = [H 2 O 2 ] [I ] The...
-
a. State the order of reaction for the decomposition of nitrogen(V) oxide. b. Use the data for 3.00 mol dm 3 N 2 O 5 in Table 22.8 to calculate a value for the rate constant for this decomposition....
-
Show that if u = tanh(x/2), then For the first relation, use the identities cosh x = 1+u 1-4 sinh x = 2u 1-u dx = 2du 1- u
-
how is lateral force(fy) determined from this data Tyre Responses 1 1 1 1 1 1.3 1.3 1.3 1.3 1.3 1.6 1.55 1.45 1.27 1.1 Fz (N) 0 400 800 1200 1500 Slip Angle (deg) Fy1 (N) Fy2 (N) Fy3 (N) 0.0 0 0 0.5...
-
(13%) Problem 8: A wire is oscillated to create a wave of the form y(x,t) = Asin(x - 30t) == The wave is reflected from a fixed end producing a reflection of the form y2(x,t) = A sin(x + 30t) The two...
-
Using the definitions of even integer and odd integer, give a proof by contraposition that this statement is true for all integers n: If 5n+3 is even, then n is odd.
-
7. Design the formwork for a wall 8-ft (2.44-m) high to be poured at the rate of 5 ft/h (1.53 m/h) at a temperature of 77F (25C). The concrete mixture will use Type I cement without retarders and is...
-
tempt in Progress The City of Minden entered into the following transactions during the year 2026. 1. A bond issue was authorized by vote to provide funds for the construction of a new municipal...
-
Glacier Creamery makes and sells ice cream. In developing the operating profit projections, the financial analyst is interested in the sensitivity to two parameters: the quantity of ice cream sold,...
-
Ann hires a nanny to watch her two children while she works at a local hospital. She pays the 19 year-old nanny $125 per week for 48 weeks during the current year. a. What is the employers portion of...
-
The Youngs modulus (see P2.40) of muscle fiber is approximately 2.80 10 7 Pa. A muscle fiber 3.25 cm in length and 0.125 cm in diameter is suspended with a mass M hanging at its end. Calculate the...
-
DNA can be modeled as an elastic rod which can be twisted or bent. Suppose a DNA molecule of length L is bent such that it lies on the arc of a circle of radius R c . The reversible work involved in...
-
A 1.75 mole sample of an ideal gas is compressed isothermally from 62.0 L to 19.0 L using a constant external pressure of 2.80 atm. Calculate q, w, U, and H.
-
Accounting changes fall into one of three categories. Identify and explain these categories and give an example of each one.
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...

Study smarter with the SolutionInn App


