The rate equation for the reaction between iodine and propanone is: rate = k[CH 3 COCH 3
Question:
The rate equation for the reaction between iodine and propanone is:
rate = k[CH3COCH3] [H+] [I2]0
a. State the order of reaction with respect to iodine.
b. State the overall order of reaction.
c. i. What is meant by the term half-life?
ii. In an experiment a large excess of iodine is reacted with a small concentration of propanone in the presence of H+(aq). The concentration of propanone is measured at regular time intervals. What happens to the value of the half-life of the propanone concentration as the concentration of propanone decreases?
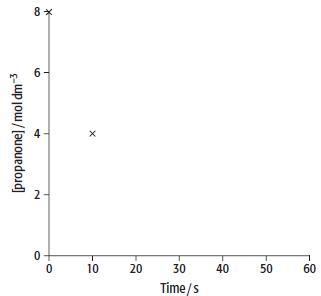
d. Copy the sketch graph. Plot additional points at 10-second intervals up to 50 s. Join all the points with a smooth curve.
e. Explain the term rate-determining step.
f. Suggest a possible mechanism for the rate-determining step for the reaction between iodine and propanone.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





