Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. In a cell, respiration reaction occurs as follows: C6H12O6+O2CO2+H2O O2 diffuses through cell membrane of 10 nm thickness to the cell surface and completely
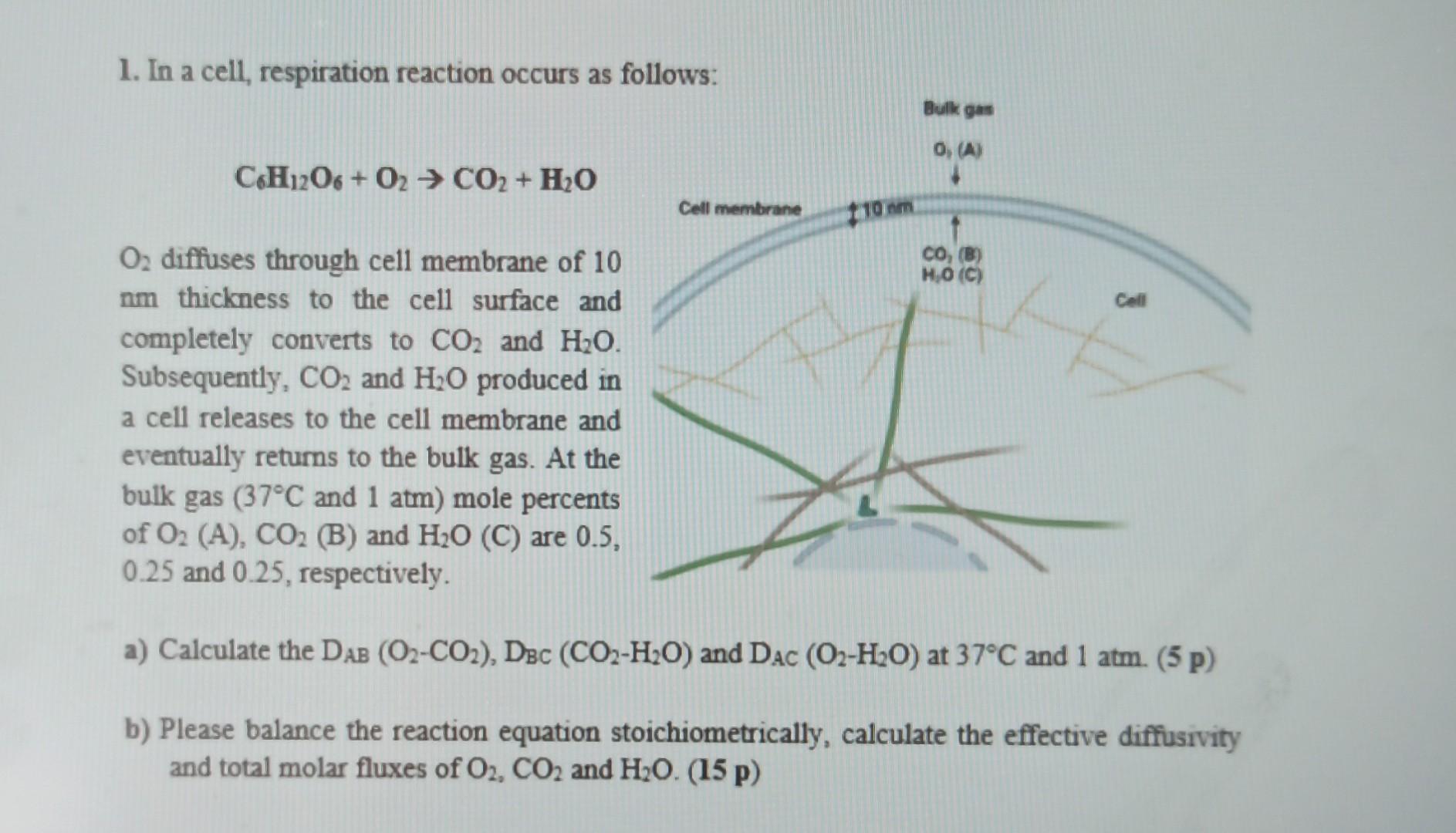
1. In a cell, respiration reaction occurs as follows: C6H12O6+O2CO2+H2O O2 diffuses through cell membrane of 10 nm thickness to the cell surface and completely converts to CO2 and H2O. Subsequently, CO2 and H2O produced in a cell releases to the cell membrane and eventually returns to the bulk gas. At the bulk gas (37C and 1atm) mole percents of O2(A),CO2(B) and H2O (C) are 0.5 , 0.25 and 0.25 , respectively. a) Calculate the DAB(O2CO2),DBC(CO2H2O) and DAC(O2H2O) at 37C and 1atm. (5 p) b) Please balance the reaction equation stoichiometrically, calculate the effective diffusivity and total molar fluxes of O2,CO2 and H2O.(15 p) 1. In a cell, respiration reaction occurs as follows: C6H12O6+O2CO2+H2O O2 diffuses through cell membrane of 10 nm thickness to the cell surface and completely converts to CO2 and H2O. Subsequently, CO2 and H2O produced in a cell releases to the cell membrane and eventually returns to the bulk gas. At the bulk gas (37C and 1atm) mole percents of O2(A),CO2(B) and H2O (C) are 0.5 , 0.25 and 0.25 , respectively. a) Calculate the DAB(O2CO2),DBC(CO2H2O) and DAC(O2H2O) at 37C and 1atm. (5 p) b) Please balance the reaction equation stoichiometrically, calculate the effective diffusivity and total molar fluxes of O2,CO2 and H2O.(15 p)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


