Question
1. Ionization State of Histidine Each ionizable group of an amino acid can exist in one of two states, charged or neutral. The electric charge
1. Ionization State of Histidine Each ionizable group of an amino acid can exist in one of two states, charged or neutral. The electric charge on the functional group is determined by the relationship between its pKa and the pH of the solution. This relationship is described by the Henderson-Hasselbalch equation.
Histidine has three ionizable functional groups. What is the net charge of histidine at pH 1, 4, 8, and 12? For each pH, will histidine migrate toward the anode (+) or toward the cathode () when placed in an electric field?
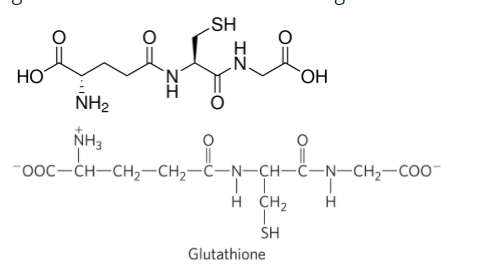
2.Glutathione is an important peptide antioxidant found in cells from bacteria to humans. Identify the three amino acid constituents of glutathione. What is unusual about glutathiones structure?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


