Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Lil reacts with O to give I and LiO in a closed vessel at constant pressure P. 2Lil+0) Li0+12) AGRON The reaction will

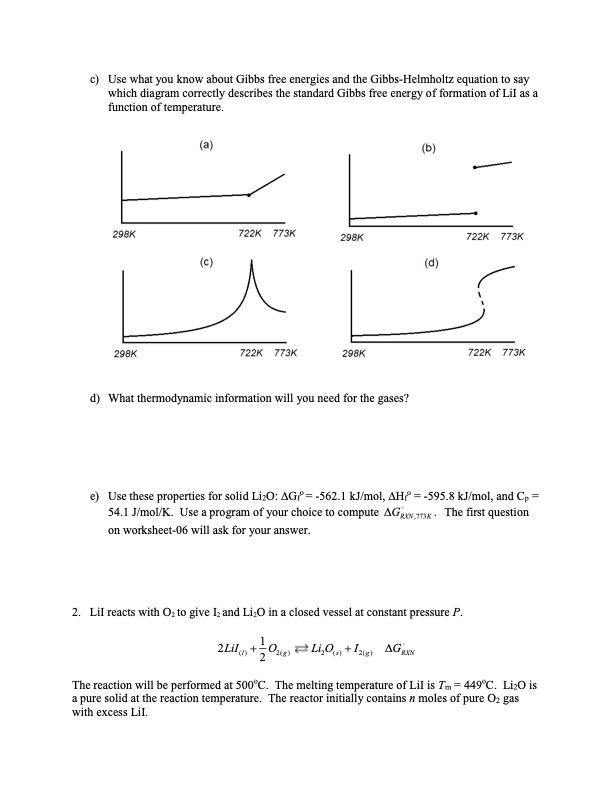

1. Lil reacts with O to give I and LiO in a closed vessel at constant pressure P. 2Lil+0) Li0+12) AGRON The reaction will be performed at 500C. The melting temperature of Lil is Tm=449C. LiO is a pure solid at the reaction temperature. The reactor initially contains n moles of pure O2 gas with excess Lil. a) You will need some unusual thermodynamic information to compute AGRXN". Which curve accurately describes the standard enthalpy of formation vs. temperature for Lil? (a) (b) 298K 298K (c) 722K 773K 298K 722K 773K 298K (d) 722K 773K 722K 773K b) Lil is a solid at the reference temperature (25C) with properties AG=-266.9 kJ/mol and AH=-270.48 kJ/mol. The heat capacity of the solid is Cp,solid 54.4 J/mol/K. Lil melts at 449C and the heat of fusion is AHm=14.63 kJ/mol. The liquid heat capacity is Cp.liquid=63.2 J/mol/K. You must integrate the Gibbs-Helmholtz equation to compute AGRXN, but first match each property to the feature it controls in the enthalpy vs. temperature diagram. Please select the correct form of plot from (a) and correspond a list of features and options (AH298K Cold Clawd and AH) to properties of the curve (i.e. intercept, slope, gap, ...). c) Use what you know about Gibbs free energies and the Gibbs-Helmholtz equation to say which diagram correctly describes the standard Gibbs free energy of formation of Lil as a function of temperature. (a) (b) 298K 722K 773K 298K (c) 298K 722K 773K 298K d) What thermodynamic information will you need for the gases? 722K 773K (d) 722K 773K e) Use these properties for solid LiO: AG=-562.1 kJ/mol, AH? =-595.8 kJ/mol, and Cp = 54.1 J/mol/K. Use a program of your choice to compute AGNK. The first question on worksheet-06 will ask for your answer. 2. Lil reacts with O to give I, and LiO in a closed vessel at constant pressure P. 2Lil + LiO +12 AGN The reaction will be performed at 500C. The melting temperature of Lil is Tm=449C. LiO is a pure solid at the reaction temperature. The reactor initially contains n moles of pure O2 gas with excess Lil. a) In the previous worksheet, you computed AGAN,773K Give your answer here in kJ/mol. b) Using K = exp[-AGS/kT] you can compute a dimensionless equilibrium constant. Construct the mass action ratio of activities and equate it to your undetermined constant K773k. Based on your expression, what units would the equilibrium constant have for those engineers who prefer to absorb the reference pressures into the equilibrium constant? c) Construct a stoichiometric table. This time use conversion. d) Find the equilibrium conversion Xo, as a function of Ko, n, P, P. e) Use the correct value of Keq to estimate the mole fraction of Oz in the gas phase at equilibrium. Let the total pressure be latm.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


