Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. LPG is a mixture of 70% butane and 30% propane by volume. The mixture is compressed from atmospheric pressure and temperature of 1
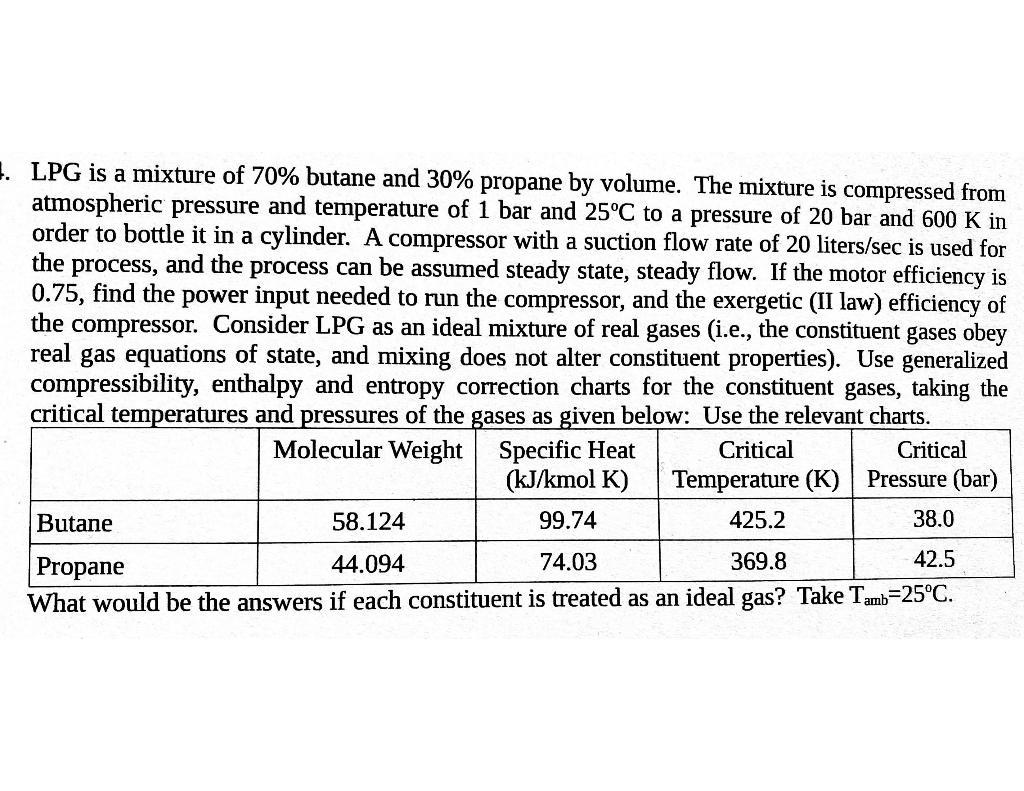
1. LPG is a mixture of 70% butane and 30% propane by volume. The mixture is compressed from atmospheric pressure and temperature of 1 bar and 25C to a pressure of 20 bar and 600 K in order to bottle it in a cylinder. A compressor with a suction flow rate of 20 liters/sec is used for the process, and the process can be assumed steady state, steady flow. If the motor efficiency is 0.75, find the power input needed to run the compressor, and the exergetic (II law) efficiency of the compressor. Consider LPG as an ideal mixture of real gases (i.e., the constituent gases obey real gas equations of state, and mixing does not alter constituent properties). Use generalized compressibility, enthalpy and entropy correction charts for the constituent gases, taking the critical temperatures and pressures of the gases as given below: Use the relevant charts. Molecular Weight Specific Heat Butane Propane 58.124 44.094 (kJ/kmol K) 99.74 Critical Temperature (K) Critical Pressure (bar) 425.2 38.0 74.03 369.8 42.5 What would be the answers if each constituent is treated as an ideal gas? Take Tamb=25C.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


