Question
1 mol of Nitrogen (N) enters a steady-state flow process at 800K and 50 bar and exits at 300K and 1 bar. Assume that
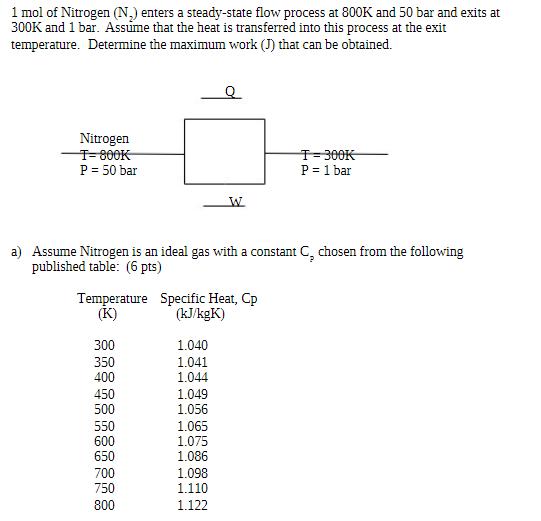
1 mol of Nitrogen (N) enters a steady-state flow process at 800K and 50 bar and exits at 300K and 1 bar. Assume that the heat is transferred into this process at the exit temperature. Determine the maximum work (J) that can be obtained. Nitrogen T-800K P = 50 bar T-300K P = 1 bar W a) Assume Nitrogen is an ideal gas with a constant C, chosen from the following published table: (6 pts) Temperature Specific Heat, Cp (K) (kJ/kgK) 300 1.040 350 1.041 400 1.044 450 1.049 500 1.056 550 1.065 600 1.075 650 1.086 700 1.098 750 1.110 800 1.122
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Analysis Synthesis And Design Of Chemical Processes
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting
5th Edition
0134177401, 978-0134177403
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



