Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1 mole of a monatomic ideal gas undergoes a cyclic process in a Heat Engine as shown in the Pressure (P) and Volume (V) diagram.
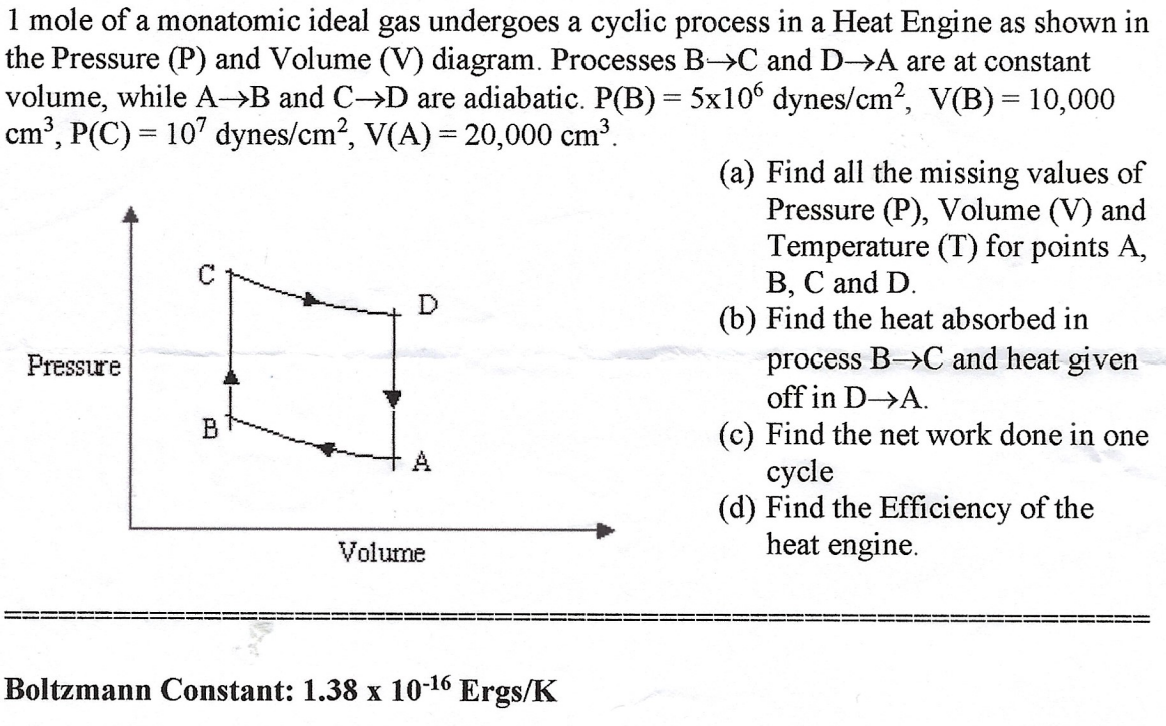 1 mole of a monatomic ideal gas undergoes a cyclic process in a Heat Engine as shown in the Pressure (P) and Volume (V) diagram. Processes BC and DA are at constant volume, while AB and CD are adiabatic. P(B)=5106 dynes /cm2,V(B)=10,000 cm3,P(C)=107 dynes /cm2,V(A)=20,000cm3 (a) Find all the missing values of Pressure (P), Volume (V) and Temperature (T) for points A, B, C and D. (b) Find the heat absorbed in process BC and heat given off in DA. (c) Find the net work done in one cycle (d) Find the Efficiency of the heat engine. Boltzmann Constant: 1.381016Ergs/K
1 mole of a monatomic ideal gas undergoes a cyclic process in a Heat Engine as shown in the Pressure (P) and Volume (V) diagram. Processes BC and DA are at constant volume, while AB and CD are adiabatic. P(B)=5106 dynes /cm2,V(B)=10,000 cm3,P(C)=107 dynes /cm2,V(A)=20,000cm3 (a) Find all the missing values of Pressure (P), Volume (V) and Temperature (T) for points A, B, C and D. (b) Find the heat absorbed in process BC and heat given off in DA. (c) Find the net work done in one cycle (d) Find the Efficiency of the heat engine. Boltzmann Constant: 1.381016Ergs/K Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


